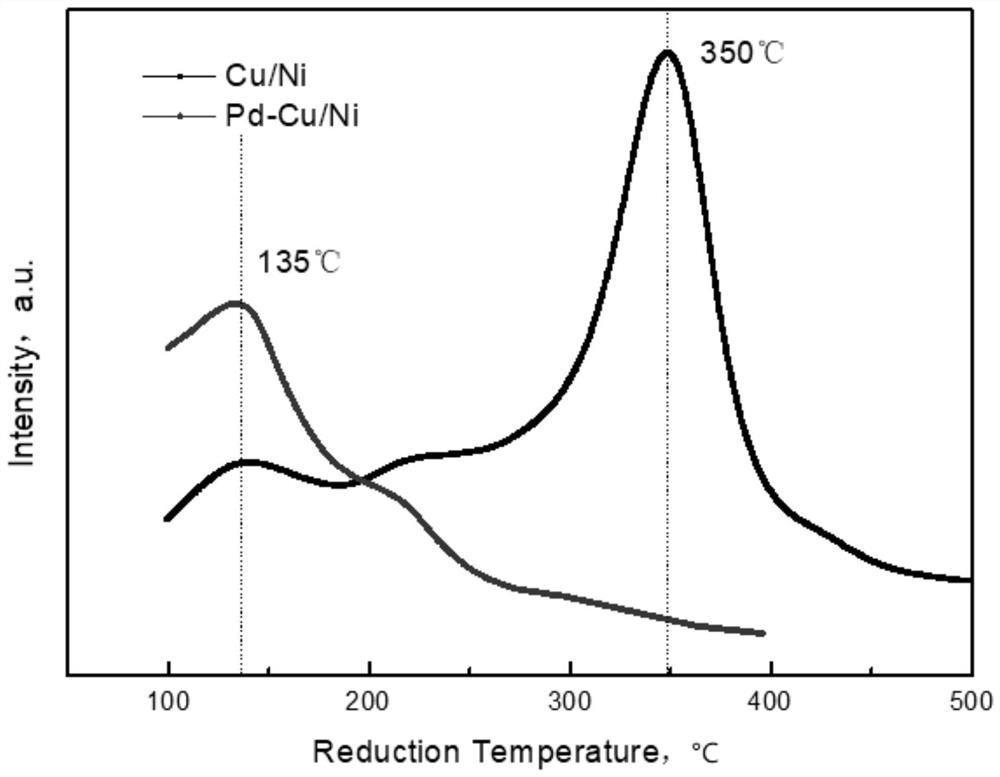
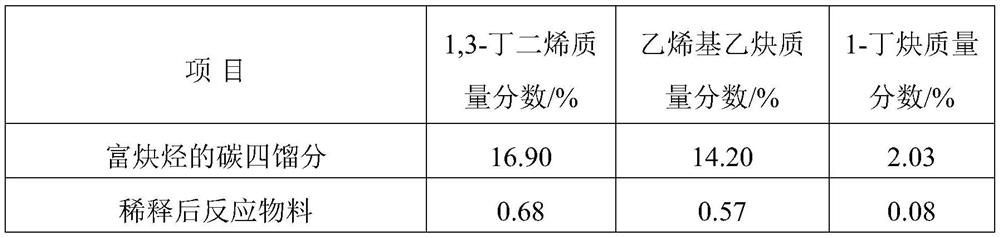
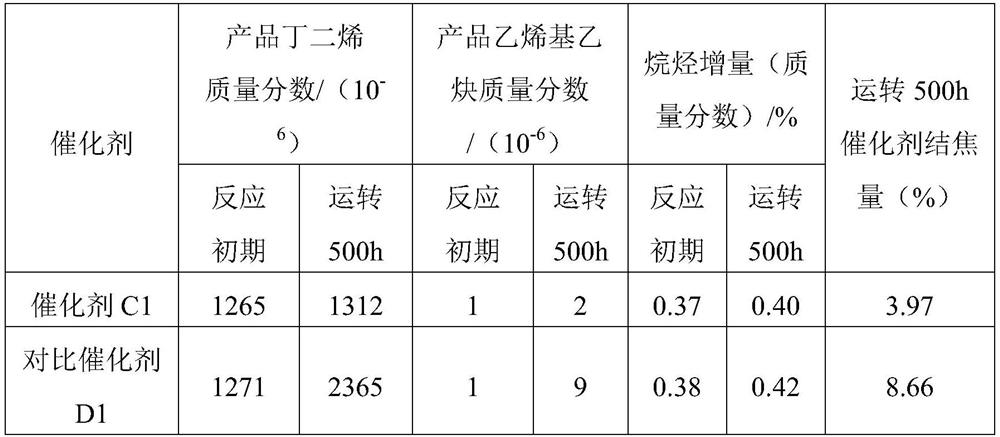
Alkyne-rich C4 fraction selective hydrogenation method
A technology for selective hydrogenation of alkyne-rich carbon four fractions, which is applied in the fields of hydrocarbons, chemical instruments and methods, hydrogenation to hydrocarbons, etc., and can solve the problems of catalyst activity decline, cost increase, selectivity decline, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Catalyst carrier prepared: 3 mm of commercially available bispacles, 3 mm diameter, after 1,100 ° C for 4 h, is 20 ~ 45 nm and 90 ~ 400 nm, respectively, the specific surface area is 43m 2 / g, weigh 100g of the carrier.
[0038] Catalyst preparation:
[0039] (1) Palladium chloride is formed into an active component impregnation liquid, and the pH is 1.7, and then the carrier is added to the salt solution of the PD, and after impregnation adsorption 2 h, 100 ° C drying for 4 h, 550 ° C, calcination 2h, and produce semi-finished catalyst C1-a.
[0040] (2) Nitrate is completely dissolved into a dipping liquid, an equal volume impregnation, and the semi-finished catalyst C1-a is immersed in the prepared solution, uniform shaking, 0.5 h, drying at 100 ° C for 2 h, 400 ° C for 6 h, Semi-finished catalyst C1-B.
[0041] (3) Nickel nickel nitrate, copper nitrate is dissolved in 70 g of water, and 35 g of n-hexane, 17 g of polyethylene glycol octylphenyl ether (Tritonx-100), 17 g...
Embodiment 2
[0055] Catalyst carrier prepared: 3 mm of commercially available bispy pores, a diameter of 3 mm, after 1050 ° C for 4 h, is 14 to 45 nm and 85 to 350 nm, respectively, the specific surface area is 60m 2 / g, weigh 100g of the carrier.
[0056] Catalyst preparation:
[0057] (1) Nickel nickel nitrate, copper nitrate is dissolved in 80 g of water, add 40 g of n-pentane, 20 g of hexadalkyltrimethyla bromide, 20 g n-pentanol thorns to form a microemulsion, and add the carrier to the prepserned microemulsion After 0.5 h in 0.5 h, the residue was filtered, washed with deionized water to neutral. It was dried at 60 ° C for 6 h, baked at 590 ° C for 2 h, and semi-finished catalyst C2-a was obtained.
[0058] (2) Palladium chloride is dissolved in 80 g of water, 40 g n-pentane, 20 g of hexadalkyltrimethylammonium bromide, 20 g n-pentanol thoroughly stirring to form a microemulsion. The semi-finished C2-A prepared by step (1) was immersed in the prepared microemulsion to be immersed in 0.5...
Embodiment 3
[0073] Catalyst carrier prepared: 3 to 4 mm in a commercially available bifurcous hole, a diameter of 3 to 4 mm, after 10 ° C for 4 h, is 30 ~ 45 nm and 100 ~ 500 nm, respectively, the specific surface area is 25m 2 / g, weigh 100g of the carrier.
[0074] Catalyst preparation:
[0075] (1) Nickel nickel nitrate, copper nitrate is dissolved in 75 g of water, add 34 g of n-heptane, 17 g of sorbitol monohydrate polyoxyethylene ether (Tween 80), 16 g n-pentanol thorns to form a microemulsion, add the carrier to After impregnation with a microemulsion for 2 h, the residue was filtered, washed with deionized water to neutral. It was dried at 80 ° C for 3 h at 500 ° C to prepare the semi-finished catalyst C3-a.
[0076] (2) Palladium chloride is prepared into an active component impregnation liquid, and the pH is 2.5, and the semi-finished catalyst C3-a prepared by step (1) is added to the salt solution of the PD, and the adsorption of the adsorption 2 h then dried at 110 ° C for 3 h, 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com