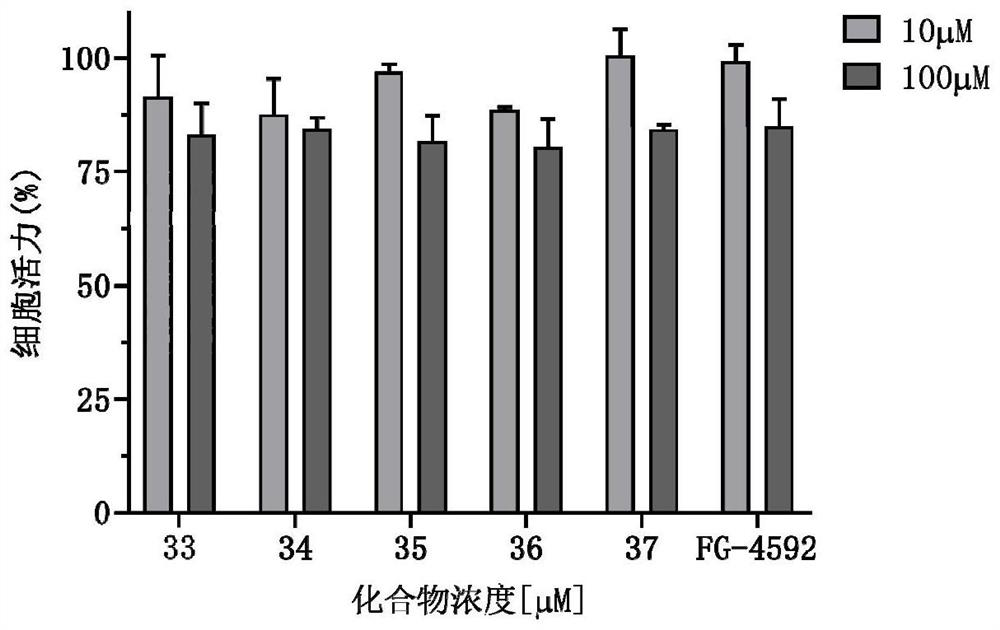
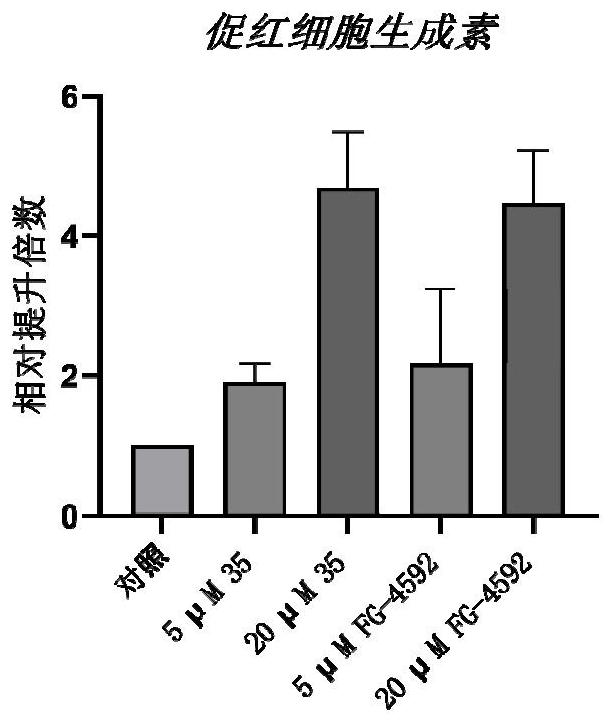
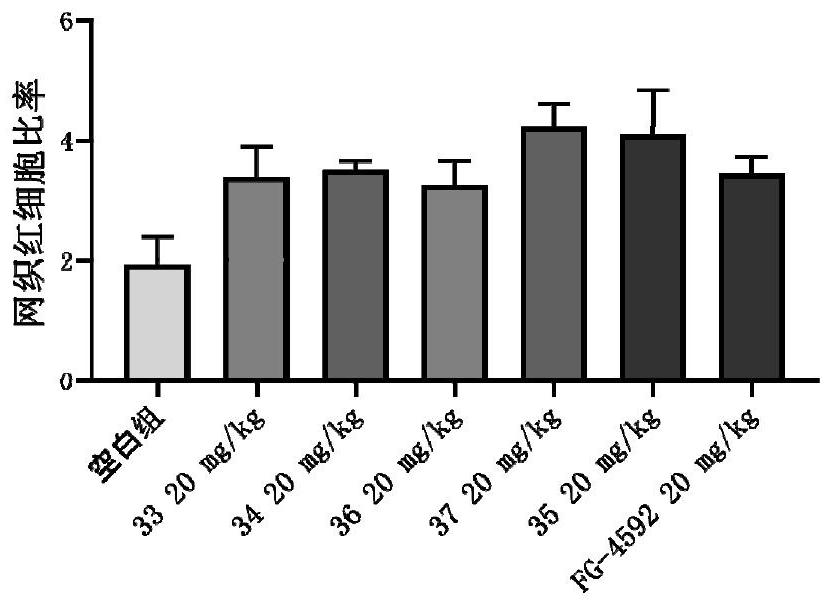
5-fat heterocyclyl-3-hydroxypyridine-2-formyl glycine compound, preparation method, pharmaceutical composition and application of 5-fat heterocyclyl-3-hydroxypyridine-2-formyl glycine compound
A technology of formylglycine and hydroxypyridine, which is applied in the direction of active ingredients of heterocyclic compounds, drug combinations, antipyretics, etc., can solve problems such as poor compliance, limited curative effect, and prone to adverse reactions, and achieves wide application and excellent preparation method Simple and feasible, excellent therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: (5-(1-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridin-4-yl)-3-benzyloxy-pyridine-2-formyl)glycine methyl ester
[0044] (3-Benzyloxy-5-bromo-3-hydroxypicolinyl)glycine methyl ester (2g, 5.27mmol) was dissolved in 40mL of 1,4-dioxane, and N-Boc-1,2,5, 6-tetrahydropyridine-4-boronic acid pinacol ester (1.79g, 5.8mmol), Pd(dppf) 2 Cl 2 (115mg, 0.158mmol), 2M Cs 2 CO 3Solution (5.16g, 15.82mmol) 10mL was heated up to 100°C and reacted under nitrogen atmosphere for 1 hour and 30 minutes. After the reaction was complete, the reaction solution was suction-filtered to remove the palladium catalyst, and the filtrate was sequentially extracted with ethyl acetate (80 mL×3) and washed with saturated sodium chloride solution (100 mL). The organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The crude product was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate ...
Embodiment 2
[0045] Example 2: (5-(1,2,5,6-tetrahydropyridin-4-yl)-3-benzyloxy-pyridine-2-formyl)glycine methyl ester
[0046] (5-(N-tert-butoxycarbonyl-1,2,5,6-tetrahydropyridin-4-yl)-3-benzyloxy-pyridine-2-formyl)glycine methyl ester (1.2g, 2.49mmol ) was dissolved in 30 mL of dichloromethane, cooled to 0°C, added trifluoroacetic acid (3.7 mL, 49.84 mmol), and reacted at room temperature for 2 hours under a nitrogen atmosphere. After the reaction was complete, ice water (10 mL) was added to quench the reaction, the reaction solution was extracted with dichloromethane (30 mL×3), and washed with saturated sodium chloride solution (30 mL). The organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. The crude product was recrystallized using a solvent (petroleum ether: ethyl acetate = 20:1) to obtain the product as an off-white powder (810mg, 85.2%). mp 113.7–114.8°C 1 H NMR (400MHz, DMSO-d6) δ8.79(t, J=6.1Hz, 1H), 8.28(d, J...
Embodiment 3
[0047] Example 3: (6-chloro-5-(1-(4-chlorophenyl)-piperidin-4-yl)-3-hydroxy-pyridine-2-formyl)glycine
[0048] (6-Chloro-5-(1-(4-chlorophenyl)-1,2,5,6-tetrahydropyridin-4-yl)-3-benzyloxy-pyridine-2-formyl)glycine methyl ester
[0049] (6-Chloro-5-(1,2,5,6-tetrahydropyridin-4-yl)-3-benzyloxy-pyridine-2-formyl)glycine methyl ester (100mg, 0.26mmol) was dissolved in 5mL To toluene, add 4-chlorobromobenzene (55mg, 0.28mmol), palladium acetate (2.94mg, 0.013mmol), BINAP (7.97mg, 0.015mmol), cesium carbonate (256mg, 0.78mmol) and react at 100°C for 10 hours. After the reaction was completed, the palladium catalyst was removed by suction filtration, and the solvent was evaporated under reduced pressure. The crude product was separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 1:2), and the product was obtained as an off-white powder (47mg, 36.4%). mp 182.4–183.7°C. 1 H NMR (400MHz, Chloroform-d) δ8.46 (d, J = 1.4Hz, 1H), 8.31 (t, J = 9.8Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com