Decoupling type carbon dioxide mineralization membrane electrolysis system for producing high-purity carbonate
A technology of carbonate and membrane electrolysis, applied in the field of electrochemistry, can solve problems such as difficult stable operation of the system, influence on system stability, attenuation, etc., and achieve the effect of continuous stable operation, low cost, and high resource conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
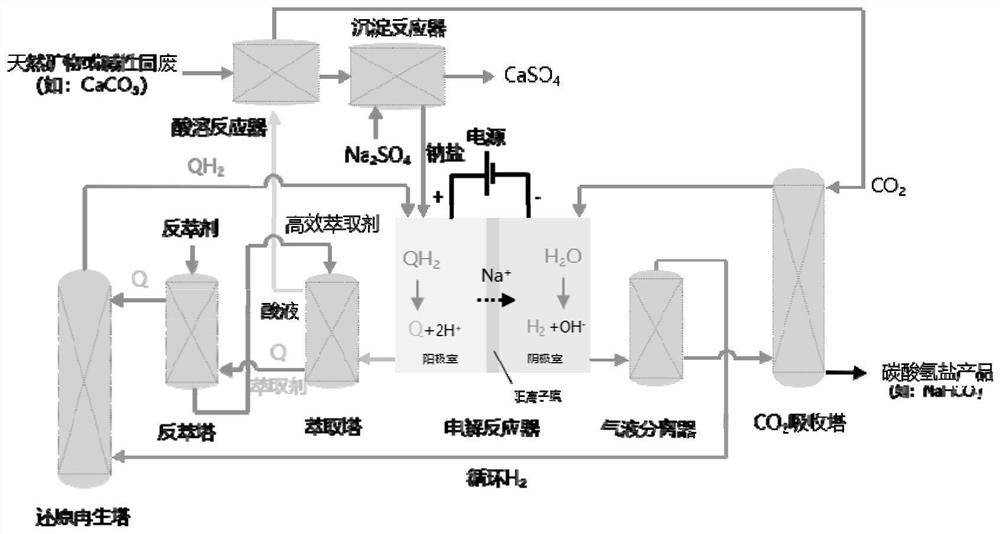
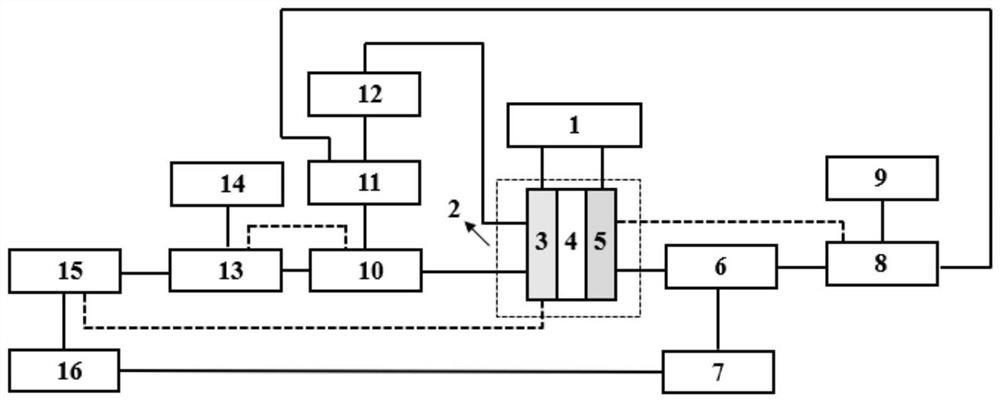
[0056] The system structure of this embodiment is as figure 2 As shown, the schematic diagram of the connection relationship of each device in the system is as described in the specific implementation, and the process is as in the attached figure 1 shown.
[0057] Using the structure in the specific embodiment to produce high-purity carbonate decoupling CO 2 Mineralized membrane electrolysis method, the specific operation is:
[0058] The cation exchange membrane Nafion 115 membrane is placed in the electrolytic cell, and the electrolytic cell is divided into a cathode area and an anode area, and 60ml of anolyte (0.01M substance ① tungstic acid derivative silicotungstic acid (SiW) + 0.5M Na 2 SO 4 ) and 60ml catholyte (1M NaHCO 3) is placed in a 200mL airtight storage tank, circulated through a pump at a flow rate of 20ml / min between the electrolyzer device, the storage tank, the extraction tower and the stripping tower, and passes into the reduction tower H 2 , the flow...
Embodiment 2
[0073] The operation process of this embodiment is basically the same as that of Example 1, the only difference is that 60ml of anolyte (leaching method to obtain red mud alkaline leaching solution+0.01M substance ② phenazine derivatives 2,3-dihydroxy-7- Phenazine sulfonate (DHPS)+0.25MNa 2 SO 4 ) and 60ml catholyte (0.5M NaHCO 3 ) is placed in a 200mL airtight storage tank, circulated through the pump between the electrolyzer device, the storage tank and the reduction tower at a flow rate of 20ml / min, and passes into the reduction tower H 2 The flow rate is 20ml / min, and the CO 2 Blow into the cathode area at a rate of 20ml / min. A DC power supply was applied between the anode electrode and the cathode electrode, and the electrolytic reaction was powered by an external DC power supply (IT6932A, Itech), and the temperature of the electrolytic cell and storage tank was set at 50 °C.
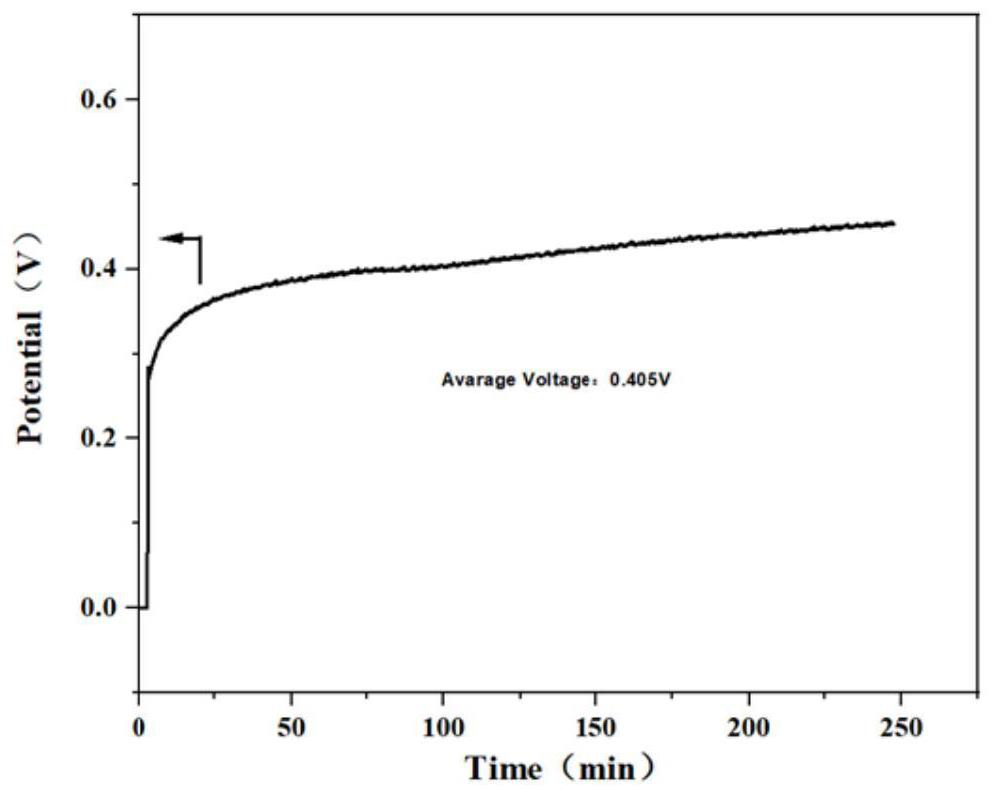
[0074] Set the current density to 10mA / cm 2 , the initial voltage is 0.181V, and the four-...
Embodiment 3
[0077] The operating process of this embodiment is basically the same as that of Example 1, the only difference being that 60ml of anolyte (the leaching method obtains red mud alkaline leaching solution+0.01M substance ③ alloxazine derivatives riboflavin (FMN)+0.5M Na 2 SO 4 ) and 60ml catholyte (1MNaHCO 3 ) is placed in a 200mL airtight storage tank, circulated through the pump between the electrolyzer device, the storage tank and the reduction tower at a flow rate of 20ml / min, and passes into the reduction tower H 2 The flow rate is 20ml / min, and the CO 2 Blow into the cathode area at a rate of 20ml / min. A DC power supply was applied between the anode electrode and the cathode electrode, and the electrolytic reaction was powered by an external DC power supply (IT6932A, Itech), and the temperature of the electrolytic cell and storage tank was set at 50 °C.
[0078] Set the current density to 10mA / cm 2 , the initial voltage is 0.15V, and the four-hour average voltage is 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com