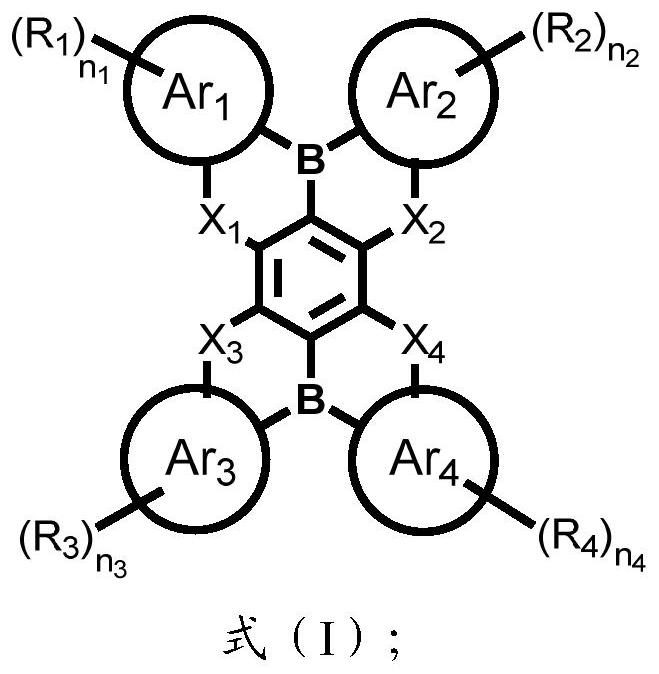
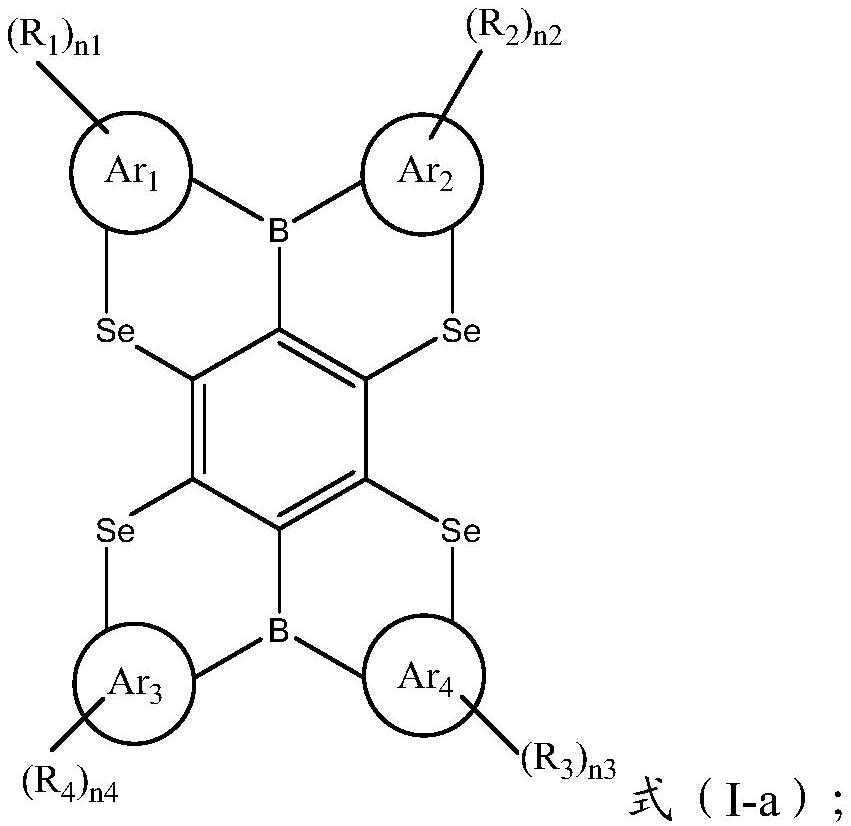
Condensed ring compound containing two boron atoms and four chalcogen atoms and organic electroluminescent device
A technology of boron atoms and compounds, which is applied to compounds containing group 3/13 elements of the periodic table, electrical solid devices, electrical components, etc., can solve problems such as complex device structures and reduced external quantum efficiency of devices, and achieve high luminous efficiency , Promote intersystem crossing, high device external quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The present invention has no special limitation on the preparation method of the condensed ring compound, and a typical preparation process thereof is as follows:
[0060]
[0061] Another typical preparation process is as follows:
[0062]
[0063] The present invention also provides an organic electroluminescent device, comprising an anode, a cathode, and an organic thin film layer between the anode and the cathode; the organic thin film layer includes the condensed ring compound shown in the above formula (I) .
[0064] The present invention has no special restrictions on the structure of the organic electroluminescent device, and conventional organic electroluminescent devices well known to those skilled in the art can be used, and those skilled in the art can select and adjust according to application conditions, quality requirements and product requirements , The structure of the organic electroluminescence device in the present invention preferably include...
Embodiment 1
[0078] The reaction formula is as follows:
[0079]
[0080] Under argon atmosphere, 1,2,4-tribromo-3,5,6-trifluorobenzene (6.7g, 18.1mmol), phenol (5.1g, 54.4mmol) and potassium carbonate ( 7.5g, 54.4mmol), add 100mL N,N-dimethylformamide (DMF) into the bottle, raise the temperature to 80°C, stir the reaction under the protection of argon for 24 hours, then cool to room temperature, pour the reaction solution into In water (1000 mL), the solid was filtered out, and the solvent was removed by suction, and the crude product was separated by column to obtain product m-1 (3.3 g, yield: 31%).
[0081] Elemental analysis structure (C 24 h 15 Br 3 o 3 ): theoretical value C, 48.77; H, 2.56 test value C, 48.62; H, 2.63.
[0082] Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS): theoretical value 587.9; experimental value 587.9.
[0083] Under argon atmosphere, add diphenyldiselenide (10.0g, 32.0mmol), sodium borohydride (2.4g, 64.0m...
Embodiment 2
[0090] The reaction formula is as follows:
[0091]
[0092] Under argon atmosphere, 1,2,4,5-tetrabromo-3,6-difluorobenzene (7.8g, 18.1mmol), p-cresol (3.9g, 36.2mmol) and Potassium carbonate (5.0g, 36.2mmol), 100mL of N,N-dimethylformamide (DMF) was added to the bottle, the temperature was raised to 80°C, and the reaction was stirred for 24 hours under the protection of argon, then cooled to room temperature, and the reaction The solution was poured into water (1000mL), the solid was filtered out, and the solvent was removed by pumping. The crude product was separated by column to obtain the product m-3 (3.6g, yield: 33%).
[0093] Elemental analysis structure (C 20 h 14 Br 4 o 2 ): theoretical value C, 39.64; H, 2.33 test value C, 39.57; H, 2.30.
[0094] MALDI-TOF-MS: theoretical value 601.8; experimental value 601.8.
[0095] Under argon atmosphere, m-4 (bis(4-methylphenyl) diselenide) (21.8g, 64.0mmol), sodium borohydride (4.8g, 128.0mmol) and 300mL DMF were adde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com