Cationic metal complex iridium-iminoquinoline crystal material and preparation method thereof
A technology of iminoquinoline and metal complexes, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., achieving the effects of short synthesis time, good thermal stability and solubility, and good economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
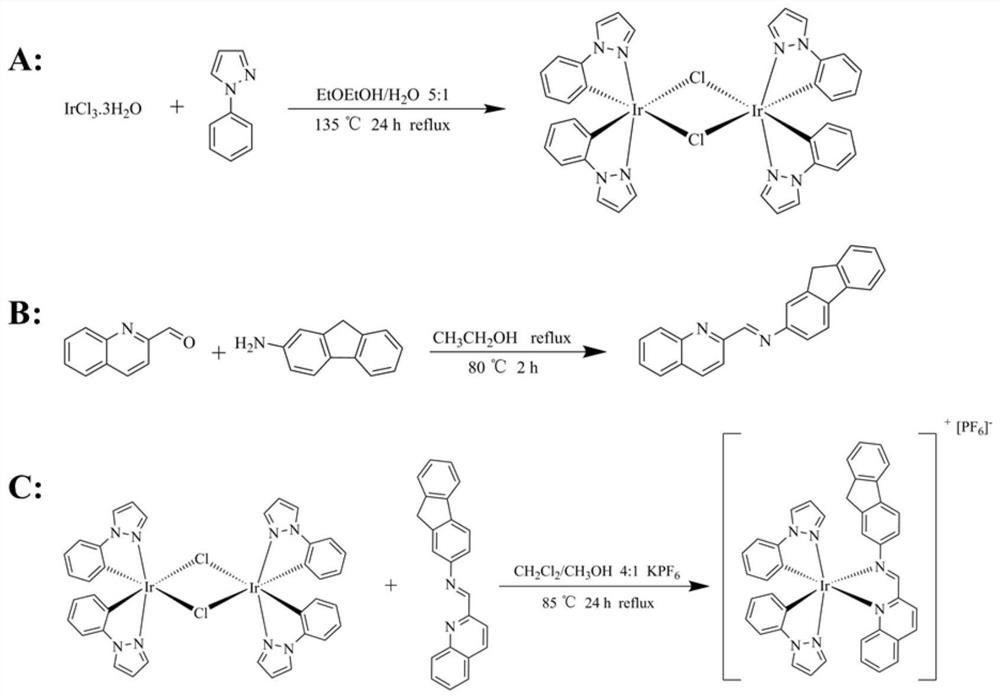
[0035] (1) Iridium dimer [Ir(ppz) 2 (μ-Cl)] 2 Synthesis:
[0036] figure 1 A is iridium dimer [Ir(ppz) 2 (μ-Cl)] 2 synthetic route map.
[0037] Add hydrated iridium trichloride 350mg (1mmol) and chelating ligand 1-phenylpyrazole 0.265mL (2mmol) in reaction bottle, then add reaction solvent ethylene glycol ether 40mL and distilled water 8mL, under nitrogen protection, in It was condensed and refluxed at 135°C for 24 hours, and the progress of the reaction was monitored by TLC during the reaction. After the reaction, the solution was cooled to room temperature and filtered with a small Buchner funnel, and the resulting precipitate was washed with ethanol and petroleum ether to remove the reaction raw materials and by-products, and then washed with CH 2 Cl 2 and H 2 O is extracted and further purified, and finally the organic phase is spin-dried to obtain 280mg of a light yellow solid [Ir(ppz) 2 (μ-Cl)] 2 (53.8%).
[0038] (2) Synthesis of auxiliary ligand 2-fluorenyl...
Embodiment 2
[0054] Step (1) and step (2) are with embodiment 1, and other steps are as follows:
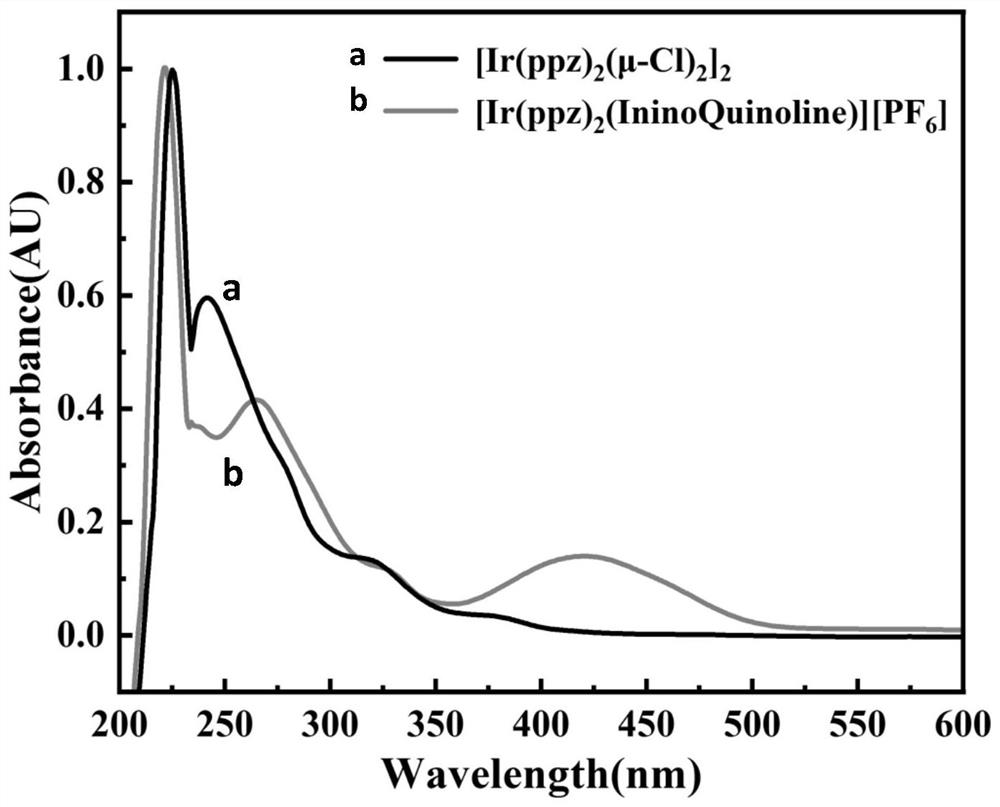
[0055] (3) Metal complex iridium-iminoquinoline [Ir(ppz) 2 (IninoQuinoline)][PF 6 ]Synthesis:
[0056] Add 103mg (0.1mmol) iridium dimer and 64.02mg (0.2mmol) auxiliary ligand 2-fluorenyliminoquinoline to a 50mL reaction flask, then add reaction solvents 30mL dichloromethane and 10mL methanol in sequence, and finally add 70 mg of potassium hexafluorophosphate was condensed and refluxed for 24 hours at 85°C in the dark under the protection of N2. After the reaction, it was purified by column chromatography (dichloromethane:petroleum ether=2:1) to obtain 52.3 mg of iridium-iminoquinoline.
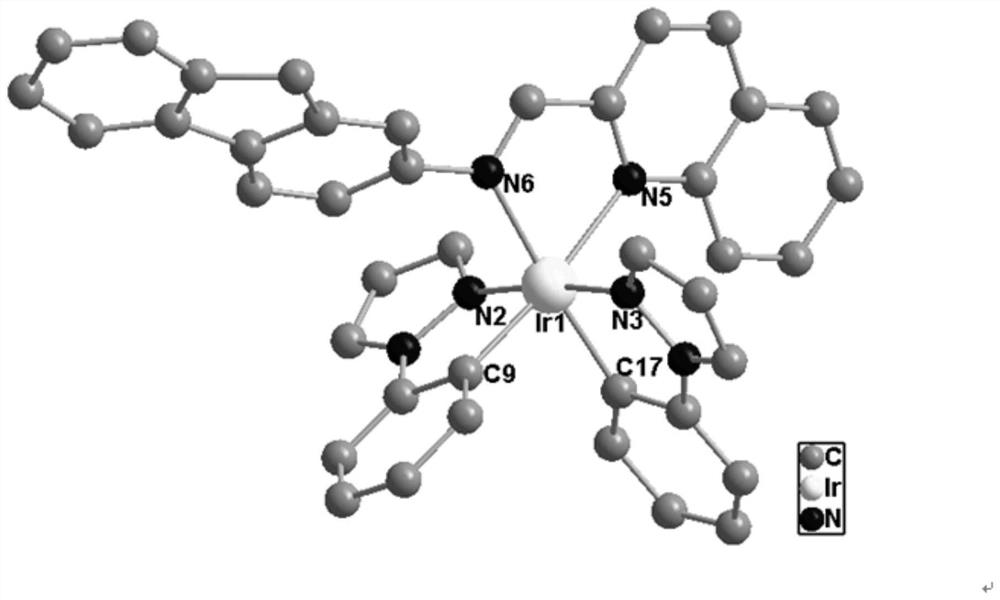
[0057] (4) Normal temperature diffusion culture of metal complex iridium-iminoquinoline crystals:
[0058] Take 1 mmol of the iridium-iminoquinoline solid and dissolve it in 1 mL of dichloromethane, then transfer the solution to a 5 mL transistor, and slowly add 1.5 mL of a buffer solution prepared by d...
Embodiment 3
[0062] Step (1) and step (2) are with embodiment 1, and other steps are as follows:
[0063] (3) Metal complex iridium-iminoquinoline [Ir(ppz) 2 (IninoQuinoline)][PF 6 ]Synthesis:
[0064] Add 103mg (0.1mmol) iridium dimer and 64.02mg (0.2mmol) auxiliary ligand 2-fluorenyliminoquinoline to a 50mL reaction flask, then add reaction solvents 20mL dichloromethane and 10mL methanol in sequence, and finally add 70 mg of potassium hexafluorophosphate was condensed and refluxed for 24 hours at 85°C in the dark under the protection of N2. After the reaction, it was purified by column chromatography (dichloromethane:petroleum ether=2:1) to obtain 51.6 mg of iridium-iminoquinoline.
[0065] (4) Normal temperature diffusion culture of metal complex iridium-iminoquinoline crystals:
[0066] Take 1 mmol of iridium-iminoquinoline solid and dissolve it in 1 mL of dichloromethane, then transfer the solution to a 5 mL transistor, then slowly add 2 mL of buffer solution prepared by dichloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com