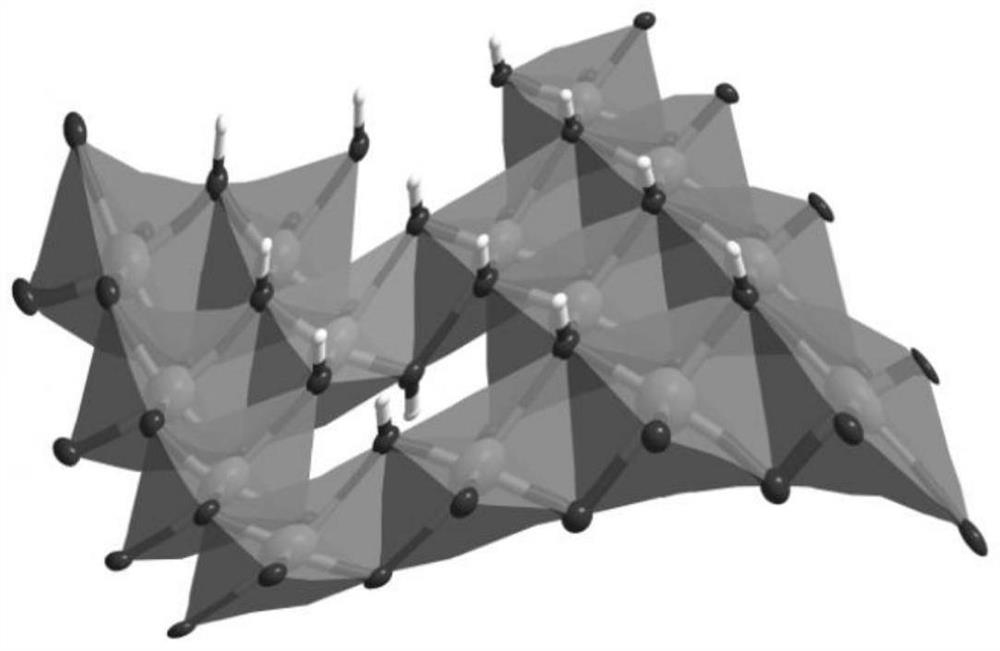
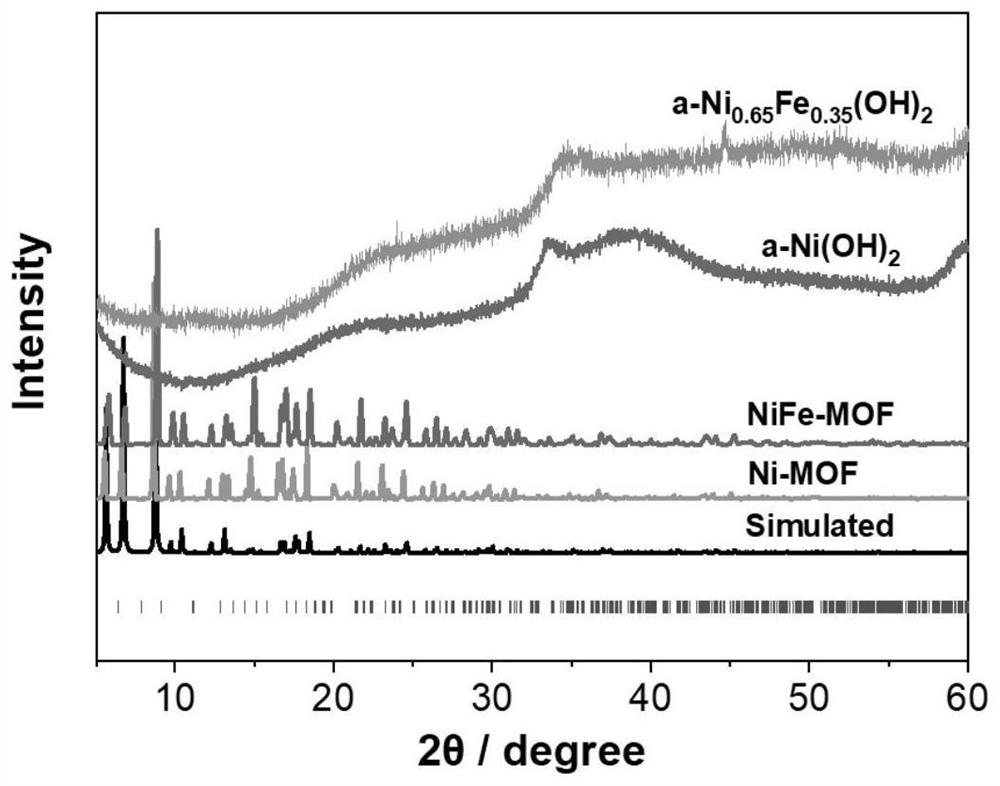
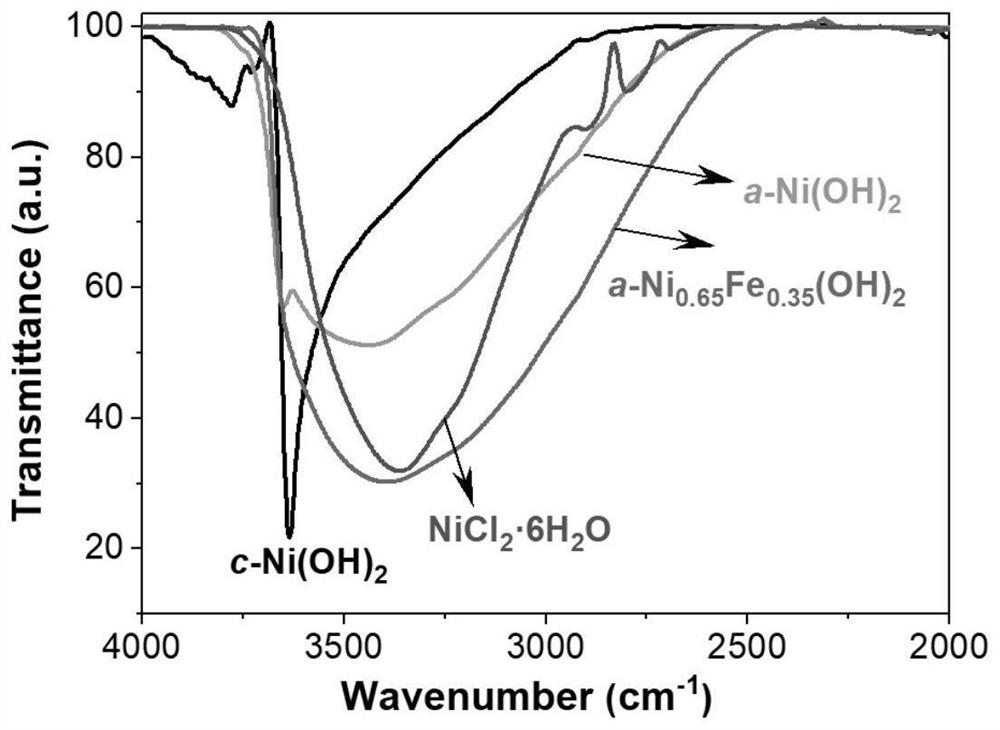
Fully-hydrolyzed amorphous hydroxide material as well as preparation method and application thereof
A hydroxide and hydrolysis technology, which is applied in the field of electrocatalysis, can solve the problems of poor electrocatalytic activity of catalysts and difficulty in realizing dual-functional full hydrolysis, and achieve easy large-scale scale-up and production, excellent electrocatalytic full hydrolysis performance, and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] A preparation method of fully hydrolyzed amorphous hydroxide material, comprising the following steps:
[0050] (1) Weigh 0.5 mmol of crystalline microporous metal-organic framework compound [Ni 3 (bpt) 2 (DMF) 2 (H 2 O) 2 ] 1.5DMF as raw material, dried and pulverized at room temperature, put into a 50mL beaker;
[0051] (2) Weigh 5mmol KOH, add 5mL aqueous solution, and configure pH=14 alkaline reaction solution;
[0052] (3) Add the alkaline reaction solution into the beaker containing the microporous metal-organic framework compound, and let it stand for reaction at room temperature for 30 minutes;
[0053] (4) After the reaction, the precipitate was collected by centrifugation and suction filtration. The precipitate was first washed with 20mL deionized water and suction-filtered 3 times in batches, and then washed with 20mL of absolute ethanol for 3 times and then dried naturally at room temperature. Dry for 3 hours, and the resulting granular solid is the fu...
Embodiment 2
[0055] (1) Weigh 0.5 mmol of crystalline microporous metal-organic framework compound [Fe 3 (bpt) 2 (DMF) 2 (H 2 O) 2 ] 1.5DMF as raw material, dried and pulverized at room temperature, put into a 50mL beaker;
[0056] (2) Weigh 5mmol NaOH, add 5mL aqueous solution, and configure pH=14 alkali reaction solution;
[0057] (3) Add the alkaline reaction solution into the beaker containing the microporous metal-organic framework compound, and let it stand for reaction at room temperature for 30 minutes;
[0058] (4) After the reaction, the precipitate was collected by centrifugation and suction filtration. The precipitate was first washed with 20mL deionized water and suction-filtered 3 times in batches, and then washed with 20mL of absolute ethanol for 3 times. After drying for 3 hours, the resulting granular solid is the fully hydrolyzed amorphous hydroxide material [Fe(OH) 2 (H 2 O) 0.6 ]·H 2 O (abbreviated as a-Fe(OH) 2 ).
Embodiment 3
[0060] (1) Weigh 0.5 mmol of crystalline microporous metal-organic framework compound [Co 3 (bpt) 2 (DMF) 2 (H 2 O) 2 ] 1.5DMF as a raw material, dried at room temperature and put into a 50mL beaker;
[0061] (2) Weigh 2.5mmol KOH, add 25mL aqueous solution, and configure pH=13 alkali reaction solution;
[0062] (3) Add the crystalline microporous metal-organic framework into the beaker containing the microporous metal-organic framework compound, and let it stand for reaction at room temperature for 60 minutes;
[0063] (4) After the reaction, the precipitate was collected by centrifugation and suction filtration. The precipitate was first washed twice with 20 mL of deionized water and filtered twice, and then washed twice with 20 mL of absolute ethanol. After drying for 3 hours, the resulting granular solid is the fully hydrolyzed amorphous hydroxide material [Co(OH) 2 (H 2 O) 0.6 ]·H 2 O (abbreviated as a-Co(OH) 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com