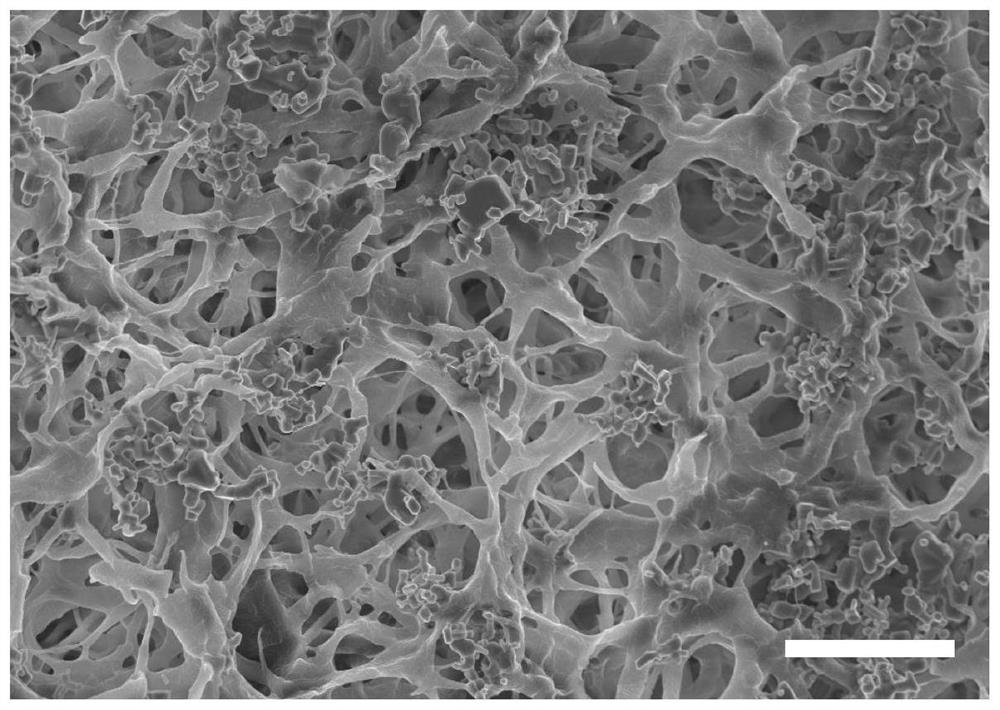
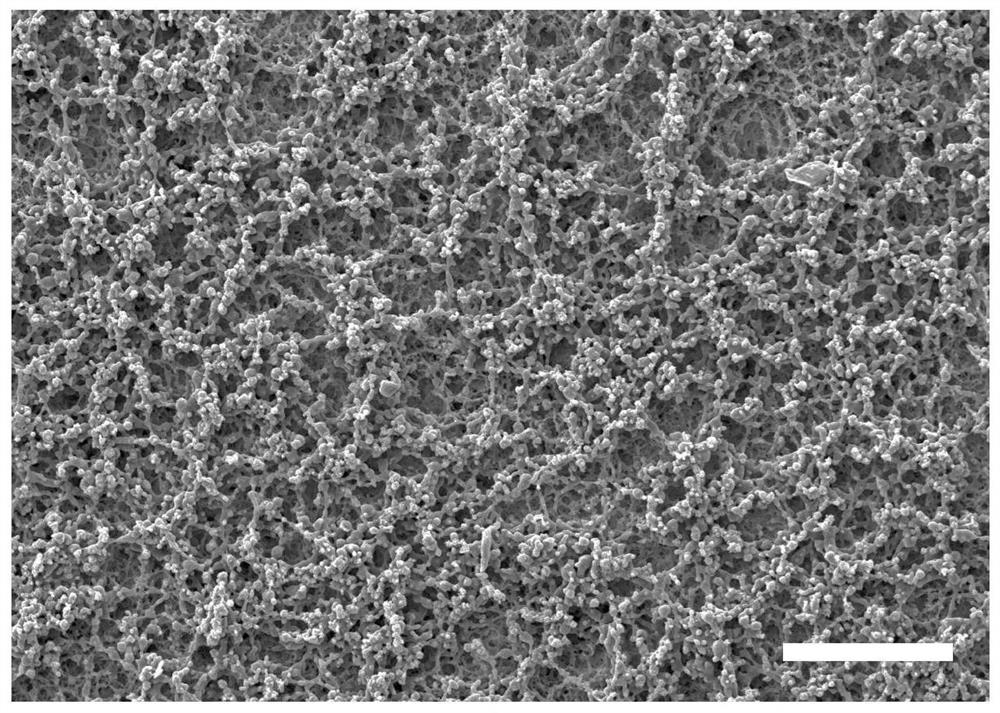
Micro-nano double-layer structure antibacterial stent as well as preparation method and application thereof
A double-layer structure, micro-nano technology, applied in the direction of antibacterial drugs, nanotechnology, nanotechnology, etc., can solve the problems of bacterial infection, low biocompatibility and low mechanical strength of polymer membranes, and achieve good antibacterial properties, Good cell compatibility, the effect of increasing the range of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a method for preparing an antimicrobial bracket with a micro-nano double-layer structure, comprising the following steps:
[0030] (1) polyhydroxyalkanoate material is mixed with nanometer antibacterial drug, add organic solvent to dissolve, obtain polyhydroxyalkanoate solution;
[0031] (2) Pour the polyhydroxyalkanoate solution into the mold, immerse the mold containing the polyhydroxyalkanoate solution in the poor solvent of the organic solvent, and perform solution replacement to obtain a semi-solid stent;
[0032] (3) After the semi-solid stent is freeze-dried, an antibacterial stent with a micro-nano double-layer structure is obtained.
[0033] In the invention, the polyhydroxyalkanoate material is detoxified and purified before preparing the antimicrobial bracket with a micro-nano double-layer structure.
[0034] In the present invention, the detoxification preferably uses a Soxhlet extractor to extract and remove endotoxins in polyhydroxy...
Embodiment 1
[0075] The PHA material used in this example is poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P34HB), which was purchased from Beijing Weimogongchang Biotechnology Co., Ltd. The nano-antibacterial drug used is nano-zinc oxide particles (diameter between 40nm and 100nm), purchased from Beijing Zhongjinyan New Material Technology Co., Ltd.
[0076] 50g of P34HB raw material was extracted by Soxhlet extractor to remove endotoxin. The extraction process is as follows: (1) Turn on the circulating water switch; (2) Set the program: use chloroform to extract and rinse at a temperature of 140°C, and the time is 3h and 2h respectively. The temperature was lowered to 40° C., the extraction cylinder was taken out, and the solvent was completely evaporated to obtain 30 g of crude extraction material of P34HB, with a loss rate of 40%. Add 300ml of chloroform to 30g of P34HB crude extraction material, spin to dissolve, and filter the solution through double-layer gauze to filter out insolu...
Embodiment 2
[0081] Cell Proliferation Experiment of Human Gingival Fibroblasts on Different Scaffolds
[0082] In a sterile environment, the P34HB+0.5% ZnO antibacterial support prepared in Example 1 was cut into a suitable circle according to the pore size of the 96-well plate and placed in the 96-well plate. When the antibacterial support was placed, the micro-pore structure layer was facing upwards Contact with cells, then inoculate human gingival fibroblasts, 5000 cells / well, in DMEM medium containing 10% fetal bovine serum (Gibco, USA) and 1% penicillin / streptomycin (Gibco, USA) at 37°C and 5%CO 2 Incubate in the incubator for 1, 4, 7 days. And with the P34HB material purified in Example 1 as a blank, the antibacterial scaffold (P34HB+1%ZnO) with a HealAll collagen film (Zhenghai Biology, China) and a nano-zinc oxide concentration of 1% prepared according to the method in Example 1 was used as a contrast ,conduct experiment. Three replicate wells were set up for each treatment and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com