Synthesis method of methyl glycolate
A technology for the synthesis of methyl glycolate and methyl glycolate, which is applied in the field of carbonylation of formaldehyde to synthesize methyl glycolate, can solve the problems of poor stability of organophosphorus ligands, high cost, difficulty in large-scale promotion and application, and achieve non-corrosive reaction equipment , high product yield, and the effect of solvent recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
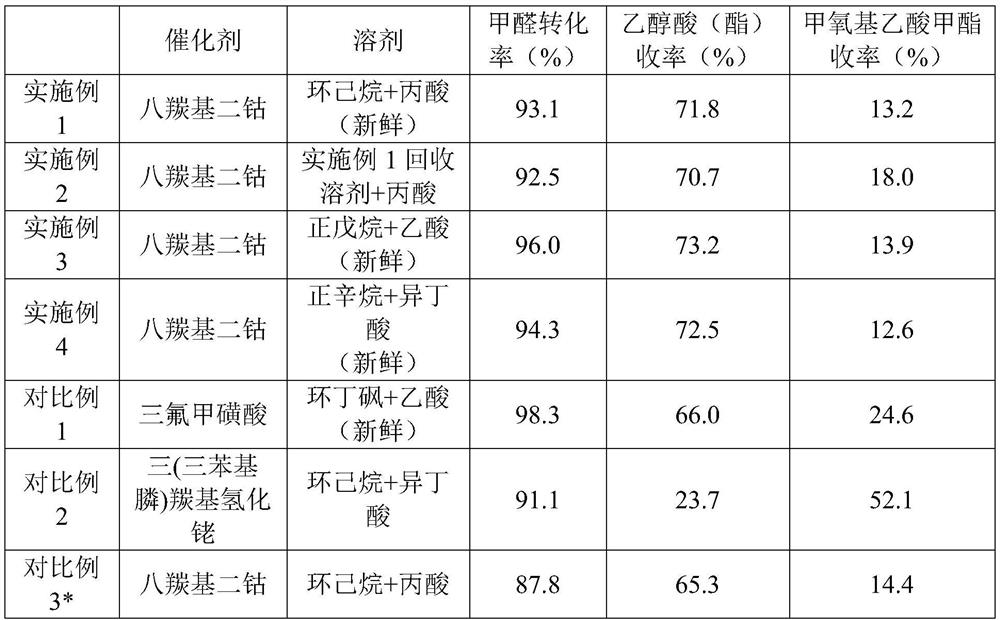
Embodiment 1
[0025] 1. Carbonylation reaction
[0026] Weigh 0.171 g (0.5 mmol) of dicobalt octacarbonyl and add it into 42 g (0.25 mol) of cyclohexane. The above homogeneous mixture was placed in a stainless steel autoclave, 0.25 g (3 mmol) of 1-methylimidazole, 18.5 g (0.25 mol) of propionic acid, and 0.25 mol of paraformaldehyde were added in sequence, and the autoclave was sealed. All the above operations were carried out in a vacuum glove box. The reactor is taken out through the transition chamber and installed on the base. The air in the kettle was replaced with CO 3 times, high-pressure CO was introduced to 8 MPa, and the reaction was carried out at 110° C. for 3 h. After the reaction, the reactor was cooled to room temperature, all the reaction solution was taken out, and the layers were separated. Filter the dark liquid in the lower layer through a microporous membrane to obtain the carbonylation product, which is set aside.
[0027] 2. Esterification reaction
[0028] Put t...
Embodiment 2
[0031] 1. Reaction solvent recovery
[0032] The upper layer of the liquid after the carbonylation reaction in Example 1 was taken out, filtered through a microporous membrane, and set aside.
[0033] 2. Carbonylation reaction
[0034] Put the filtered upper solvent into a stainless steel autoclave. Add 0.5 mmol of dicobalt octacarbonyl, 0.25 g (3 mmol) of 1-methylimidazole, 18.5 g (0.25 mol) of propionic acid, and 0.25 mol of paraformaldehyde in sequence, and seal the reaction vessel. All the above operations were carried out in a vacuum glove box. The reactor is taken out through the transition chamber and installed on the base. Add CO to replace the air in the kettle for 3 times, pass high-pressure CO to 8MPa, and react at 110°C for 3h. After the reaction, the reactor was cooled to room temperature, all the reaction solution was taken out and placed in a separatory funnel, and allowed to stand until layers were separated. Filter the dark liquid in the lower layer throu...
Embodiment 3
[0039] 1. Carbonylation reaction
[0040]Weigh 0.684 g (2.0 mmol) of dicobalt octacarbonyl and add it into 101 g (1.4 mol) of n-pentane. The above homogeneous mixture was placed in a stainless steel autoclave, 1.54 g (16 mmol) of ethylimidazole, 10.2 g (0.17 mol) of acetic acid, and 0.25 mol of paraformaldehyde were added in sequence, and the autoclave was sealed. All the above operations were carried out in a vacuum glove box. The reactor is taken out through the transition chamber and installed on the base. The air in the kettle was replaced with CO for 3 times, high-pressure CO was introduced to 6MPa, and the reaction was carried out at 90°C for 5h. After the reaction, the reactor was cooled to room temperature, all the reaction solution was taken out, and the layers were separated. Filter the dark liquid in the lower layer through a microporous membrane to obtain the carbonylation product, which is set aside.
[0041] 2. Esterification reaction
[0042] Put the filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com