Application of moutan bark glycoside C in preparation of protein disulfide bond isomerase inhibitor
A technology of disulfide bond isomerase and protein, which is applied in the field of biomedicine to achieve the effect of inhibiting collagen-induced platelet aggregation and efficiently inhibiting the activity of protein disulfide bond isomerase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Screening for a small molecule inhibitor of protein disulfide bond isomerase.
[0030] experimental method:
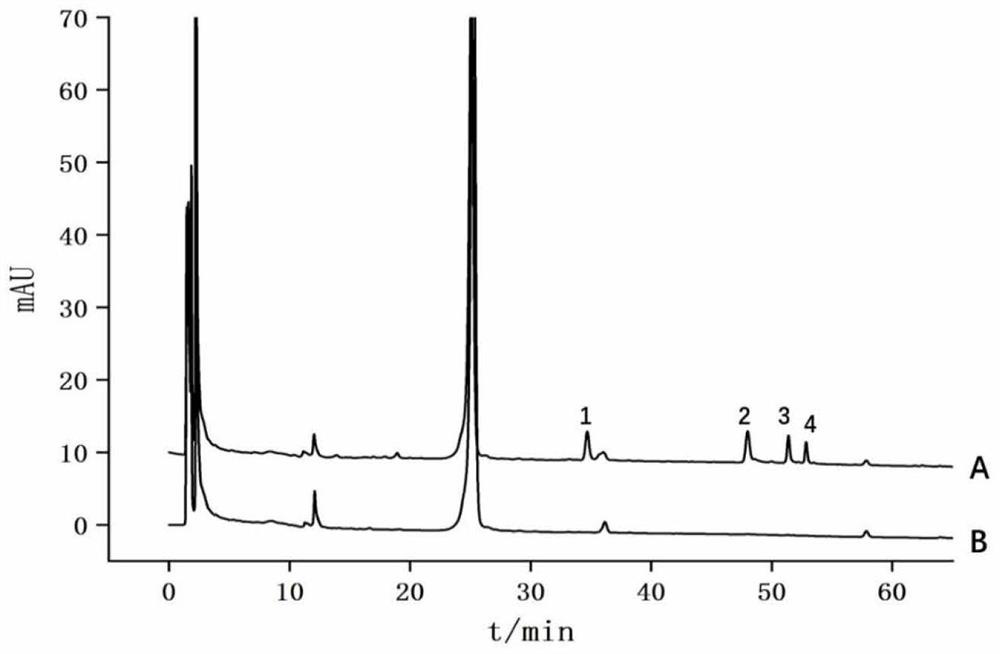
[0031] (1) Dried Moutan cortex Chinese medicine decoction pieces at below 50°C, crushed and passed through a No. 4 sieve in the Chinese Pharmacopoeia, and took the sieved Moutan cortex medicinal material powder for later use.
[0032] (2) Add 10 times the weight of ethanol with a weight percentage concentration of 75% to the Moutan Cortex powder, conduct ultrasonic extraction at 20° C. for 30 min, and the ultrasonic frequency is 40 KHz. Centrifuge at 12000rpm for 15min, take the supernatant into an evaporating dish, evaporate it to dryness at 50°C, add 1ml DMSO to redissolve, and prepare the Cortex Moutan extract with a crude drug amount of 1g / ml for future use.
[0033] (3) Dilute the product of step (2) to 10mg / ml, add 0.5ml of the diluted solution to the PDI affinity column, and incubate at 20°C for 2h. After incubation, the "unbound fraction" and the "boun...
Embodiment 2
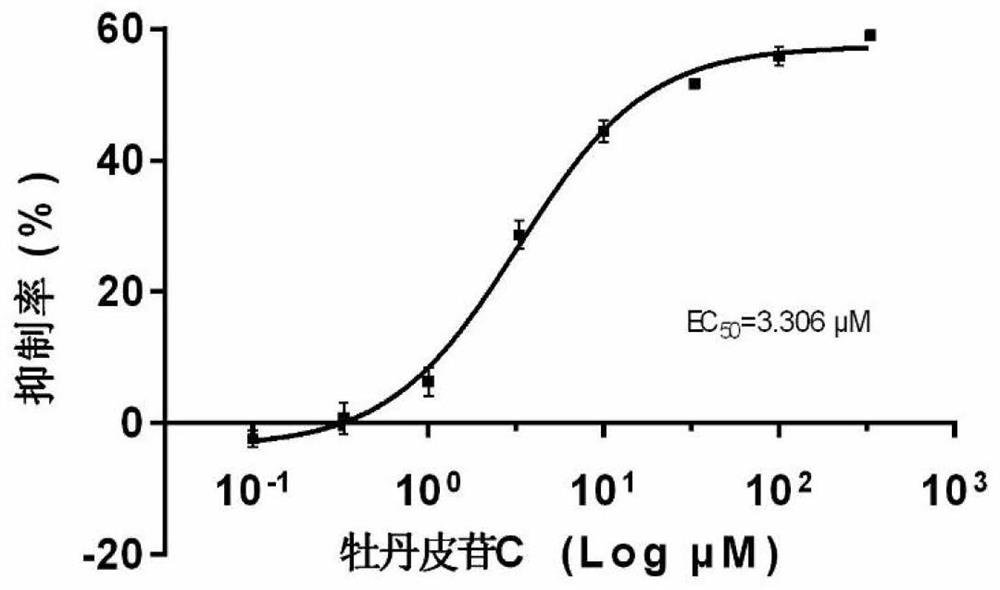
[0038] Evaluation of the inhibitory effect of a compound of the present invention on protein disulfide bond isomerase.
[0039] Experimental method: Insulin turbidimetry was used to determine the inhibitory activity of compounds on disulfide bond isomerase. Insulin consists of two peptide chains, A and B, connected by two disulfide bonds. In the presence of dithiothreitol (DTT), disulfide bond isomerase can play its role as a reductase to reduce the disulfide bond between the two peptide chains of insulin, and separate the A chain and the B chain. The large B chain will settle down, causing the reaction system to be turbid. Use a microplate reader to measure the turbidity of the reaction system to determine the activity of disulfide bond isomerase.
[0040]In the reaction system, the final concentration of disulfide isomerase is 263nM, the final concentration of insulin is 0.2mM, and the final concentration of DTT is 0.3mM. The reaction is carried out in 100mM potassium phosp...
Embodiment 3
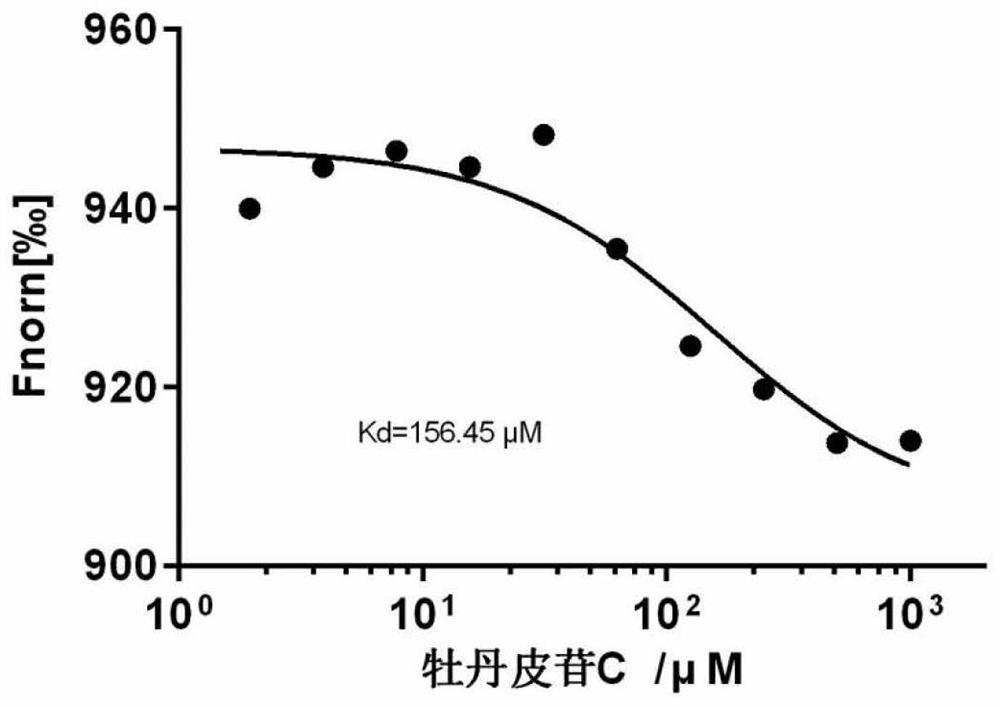
[0046] An analysis of the interaction between a compound of the present invention and protein disulfide bond isomerase.
[0047] Experimental method: use micro-scale thermophoresis analysis (Micro-Scale Thermophoresis, MST), the micro-thermophoresis instrument heats the solution in the capillary by infrared laser, the fluorescent molecules in the solution are subjected to the effect of thermophoresis to present a temperature gradient field, due to the fluorescence The degree of change in the signal is related to the binding of the ligand and the fluorescent molecule, so MST can be used to measure the intermolecular affinity.
[0048] The affinity of fluorescently labeled protein disulfide bond isomerase and its b'-x fragment to paeonidin C was determined at room temperature using MO NT.115 microthermophoresis instrument. His-tagged proteins were labeled using the MO-L018 RED-tris-NTA kit. Dilute the small molecule ligand (paeonidin C) in a gradient in a PCR tube, add the labe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com