Synthesis method of diatrizoic acid
A synthesis method, the technology of diatrizoic acid, which is applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve the problems of low resin recovery rate, three wastes, energy consumption, and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
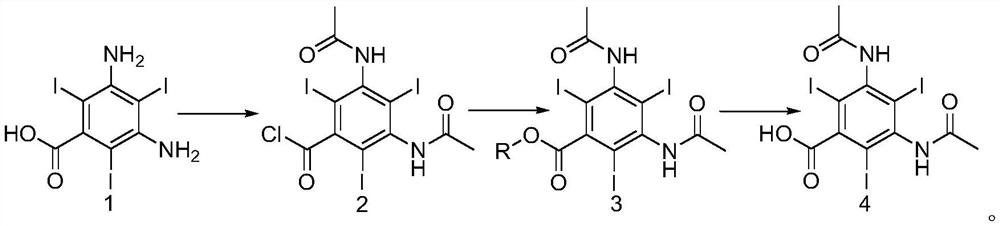
[0032] Example 1: Preparation of 3,5-diacetylamino-2,4,6-triiodobenzoyl chloride
[0033] Add 30g of 3,5-diamino 2,4,6-triiodobenzoic acid (0.0566mol) into the reaction flask, add 30g of acetic acid (0.5mol) and heat up to 50°C to dissolve, then transfer to the dropping funnel for later use 300ml (4.13mol) thionyl chloride is added in the reaction flask, 0.5g of 4-dimethylaminopyridine is added as a catalyst, stirred at 50° C., the solution in the dropping funnel is added dropwise into the reaction flask, and the dropping speed Control the internal temperature not to exceed 60°C. After the dropwise addition, keep the reaction for 24 hours, and then reduce the pressure to 60°C to evaporate the solvent in the reaction bottle to obtain about 40 g of oil.
[0034] In embodiment 1, 4-dimethylaminopyridine can be replaced by dimethylformamide, N,N-dimethylaniline or pyridine; Thionyl chloride here also acts as a solvent, if other solvents are selected, such as chloroform, two Oxyhe...
Embodiment 2
[0035] Embodiment 2: the preparation of 3,5-diacetamido-2,4,6-triiodobenzoic acid methyl ester
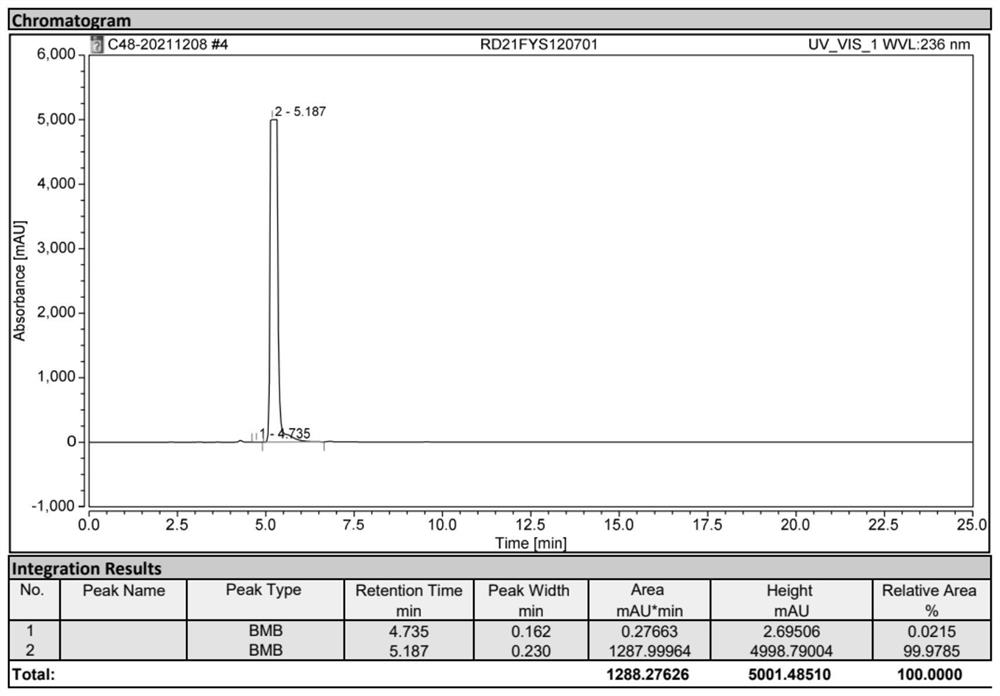
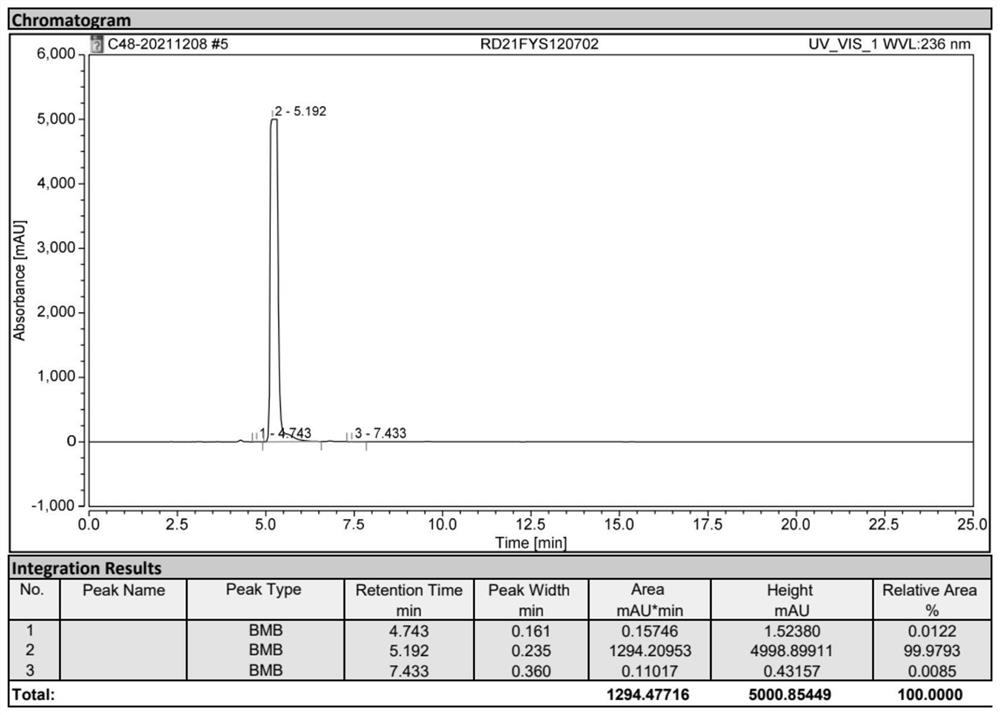
[0036] Add 40g of the 3,5-diacetylamino-2,4,6-triiodobenzoyl chloride oil prepared above into the reaction bottle, add 300ml of methanol, heat up to reflux, reflux and stir for 12h, and control the acid chloride in HPLC Stop the reaction when the compound is less than 0.5%. At this time, a large amount of white solid precipitates out. Cool down to 20-25°C to crystallize for 6 hours, filter with suction, rinse the filter cake with 10ml of methanol three times, and dry the solid to obtain 3,5-diacetylamino -2,4,6-triiodobenzoic acid methyl ester 33g (0.0525mol), molar yield 92.76% (calculated based on compound 1: 3,5-diamino-2,4,6-triiodobenzoic acid), HPLC Content 99.1%.
[0037] Methyl alcohol in embodiment 2 can be replaced with ethanol, Virahol, n-propanol, n-butanol or tert-butanol, can obtain corresponding formate compound like this.
Embodiment 3
[0038] Embodiment 3: the preparation of 3,5-diacetamido-2,4,6-triiodobenzoic acid
[0039]Add the above 33g of 3,5-diacetylamino-2,4,6-triiodobenzoic acid methyl ester into the reaction flask, add 200ml of deionized water, stir for 30min, then add 20% sodium hydroxide aqueous solution dropwise, Add dropwise to the reaction bottle to completely dissolve, adjust the pH to 10-11, maintain the pH, keep the temperature at 50-55°C for 2 hours, control the content of methyl ester compounds in HPLC to not more than 0.5%, and use concentrated hydrochloric acid to adjust the pH to 3 -3.5, stir and heat up to 90-95°C, add 0.15g of activated carbon, continue to stir for 3h, filter while hot, after the filtrate cools to 60°C, add concentrated hydrochloric acid dropwise to adjust the pH to 1-1.5, keep stirring and crystallize for 3h, pump filter, the filter cake was rinsed 3 times with 10ml deionized water, and dried to obtain 30g (0.0489mol) of diatrizoic acid crude product, with a molar y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com