A kind of method that utilizes magnesite to prepare magnesium aluminum hydrotalcite
A magnesite, nano-hydrotalcite technology, applied in the directions of hydrotalcite, chemical instruments and methods, nanotechnology for materials and surface science, etc., can solve the problem of large consumption of nitric acid and NaOH waste liquid, incomplete precipitation of magnesium sulfate, Consume large acid and ammonia water, etc., to increase the effective contact area, avoid high temperature and high pressure reaction, and achieve the effect of small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
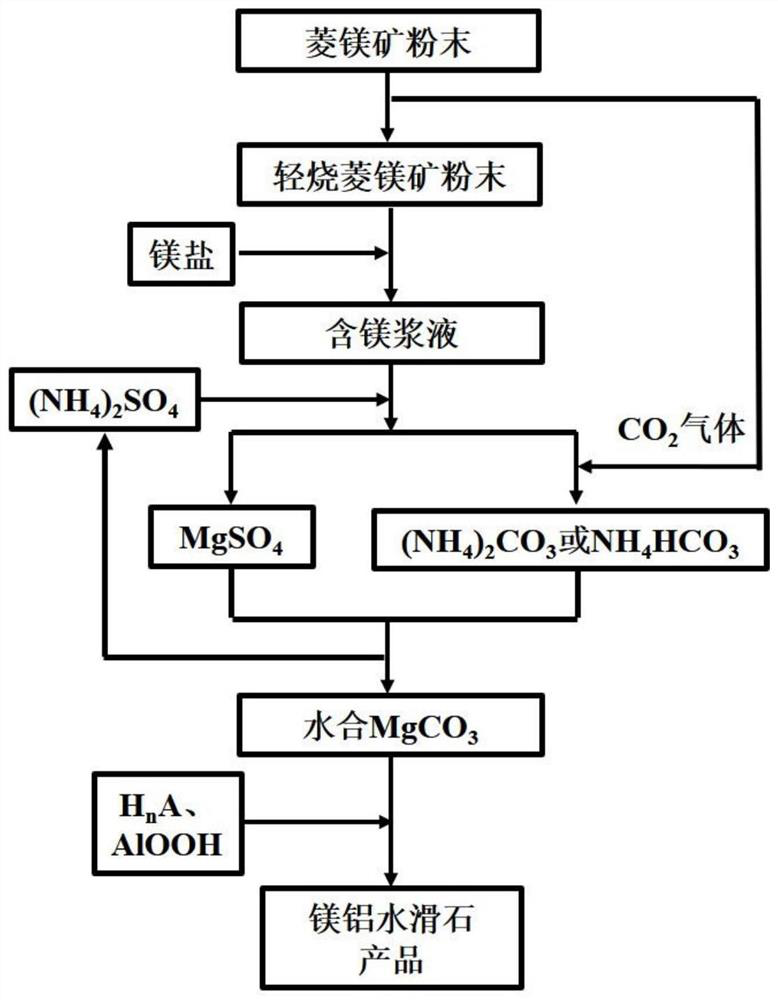
[0029] Step A: Place 1 kg of magnesite powder (calcium-magnesium molar ratio is 8%) in a muffle furnace, heat to 700° C. for calcination for 2 hours to obtain light-burned magnesite powder; 89 grams of light-burned magnesite Powder (containing 8.96 grams of CaO), 32.6 grams of magnesium chloride hexahydrate and 1 kilogram of deionized water were mixed, and ground with a colloid mill for 10 minutes to obtain a slurry; the slurry was heated to 70 ° C, stirred and reacted for 3 hours, filtered and washed to obtain Mg(OH) 2 Wet cake; Mg(OH) 2 The wet cake was added to 800 g of deionized water and ground with a colloid mill for 4 minutes to obtain Mg(OH) 2 slurry.
[0030] Step B: Combine 286 g (NH 4 ) 2 SO 4 added to Mg(OH) 2 In the slurry, heated to 100 °C, stirring to react to Mg(OH) 2 All dissolved, the solution was filtered to obtain MgSO 4 solution, the resulting ammonia vapor is condensed by a condensing device and combined with CO 2 The gas reacts to give (NH 4 ) ...
Embodiment 2
[0034] Steps A, B and C are the same as in Example 1.
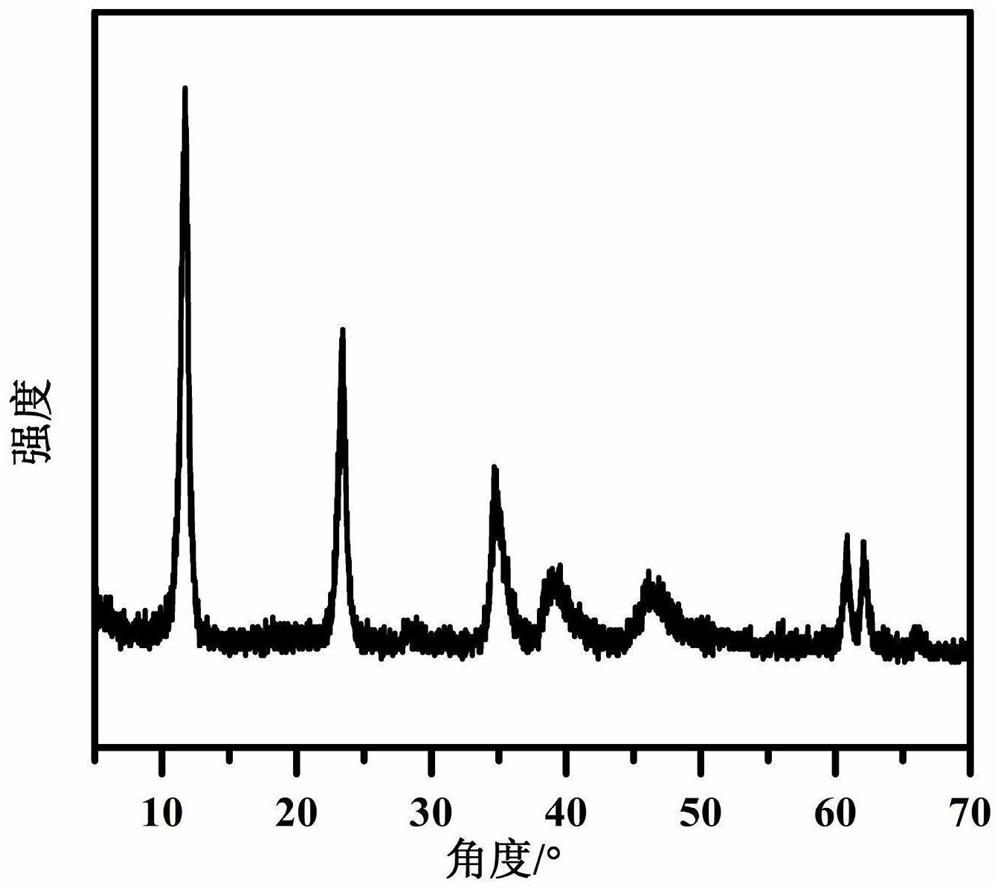
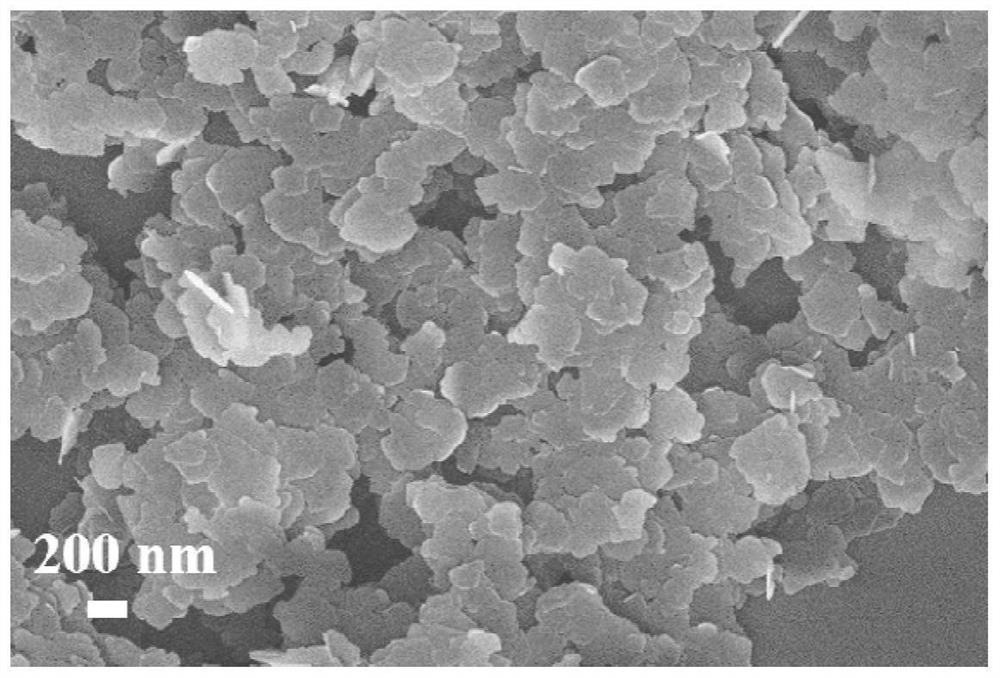
[0035] Step D: the nano-AlOOH wet filter cake (dry weight 30 grams), the hydrated MgCO obtained in step D 3 The wet filter cake and 30 g of acetic acid were added to 1.2 kg of deionized water and ground with a colloid mill for 5 minutes. The slurry was heated to 100°C and reacted under vigorous stirring for 0.5 and 1 hour, respectively. The reaction slurry was ground with a colloid mill for 6 minutes, and the reaction was continued. After 3 hours, 8 grams of lauric acid were added to modify the hydrotalcite, and after stirring for 2 hours, the precipitate was filtered and dried to obtain a lauric acid-modified nano-magnesium-aluminum hydrotalcite [Mg 0.8 Al 0.2 (OH) 2 ](CH 3 COO) 0.2 ·0.6H 2 O.
Embodiment 3
[0037] Steps A, B and C are the same as in Example 1.
[0038] Step D: the nano-AlOOH wet filter cake (dry weight 40 grams), the hydrated MgCO obtained in step C 3 Wet cake and 78.7 g of succinic acid (C 4 H 6 O 4 ) was added to 1.5 kg of deionized water and ground with a colloid mill for 8 minutes, heated to 105°C, and the reaction slurry was ground with a colloid mill for 8 minutes after reacting under vigorous stirring for 0.5 hours, and 10 grams of dodecylbenzene was added after continuing the reaction for 2.5 hours. The hydrotalcite was modified by sulfonic acid, and after stirring for 1 hour, the precipitate was filtered and dried to obtain nano-magnesium-aluminum hydrotalcite modified by dodecylbenzenesulfonic acid [Mg 0.75 Al 0.25 (OH) 2 ](C 4 H 4 O 4 ) 0.125 ·0.75H 2 O.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com