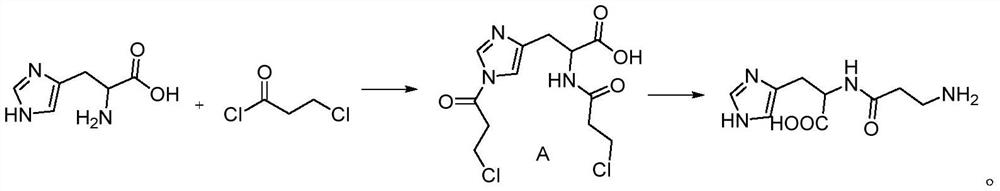
A kind of synthetic method of carnosine
A synthesis method and carnosine technology, applied in the field of organic synthesis, can solve the problems of low yield, high cost, long route and the like, and achieve the effects of short synthesis route, reduction of raw material cost, and reduction of environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
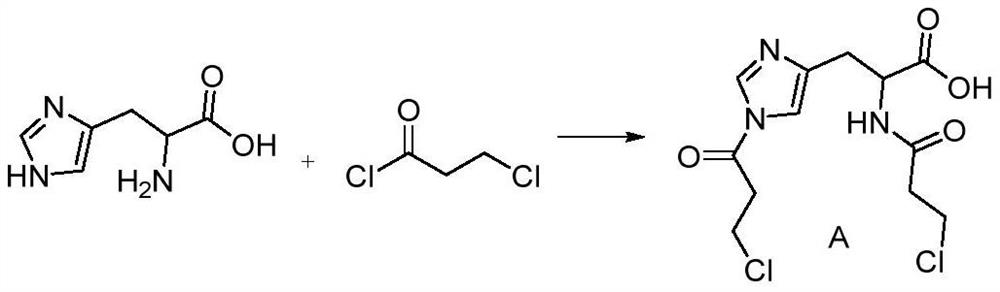
[0035] Step 1: Add 15g (0.0620mol, 1eq) L-histidine to 10mL tetrahydrofuran and 50mL water at 0-5°C, add 26g (1.5476mol, 5eq) sodium bicarbonate, stir for 30min, slowly add 20g ( 0.3937mol, 2.5eq) 3-chloropropionyl chloride, after the dropwise addition, keep it warm at 0-5°C for 5 hours, follow the complete reaction of L-histidine by TLC, let stand for 30 minutes to separate the phases, and wash the tetrahydrofuran phase with 50mL saturated saline. Dry over anhydrous sodium sulfate, evaporate to dryness under reduced pressure below 50°C to obtain a yellow oil, and obtain 18.5 g (0.0552 mol) g of compound A through column chromatography, with a molar yield of 89%.
[0036] Characterization data: 1 HNMR(400MHz,MeOD):δ8.12(s,1H),6.51(s,1H),4.45-4.49(m,1H),3.76(t,4H),3.31-3.36(dd,2H),2.86( t,4H); 13 CNMR (400MHz, MeOD): 172.3188, 171.5703, 169.5647, 131.9984, 131.5282, 117.3620, 41.0465, 40.7454, 40.3309, 40.1765, 40.0269, 31.2832.
[0037]
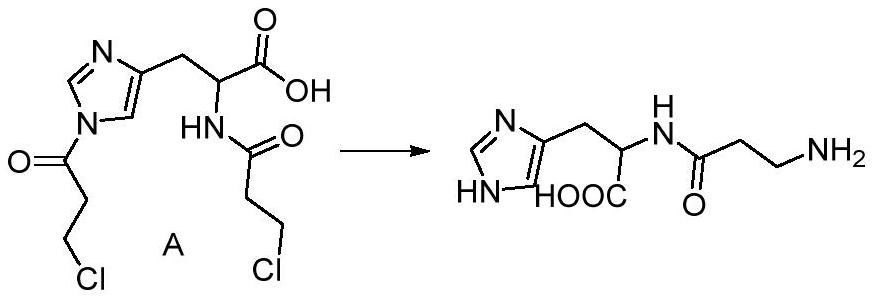
[0038] Step 2: Add 1...
Embodiment 2
[0041] Step 1: Add 5kg (32.26mol, 1eq) L-histidine to 1L tetrahydrofuran and 10L water at 0-5°C, add 2.168kg (8eq) sodium bicarbonate, stir for 120min, slowly add 20.48kg (5eq) ) 3-chloropropionyl chloride, after the dropwise addition, keep the reaction at 0-5°C for 10 hours, TLC traces the complete reaction of L-histidine, let it stand for 60 minutes to separate the phases, wash the tetrahydrofuran phase with 5L saturated saline, and dry over anhydrous sodium sulfate , Evaporated to dryness under reduced pressure below 50°C to obtain Compound A as a yellow oil.
[0042] The second step: Add the compound A obtained in the first step into the high-pressure reactor, add 95L of 20wt% ammonia water, vacuumize and fill the ammonia gas to 0.5MPa, heat to 60°C, keep the temperature for 18 hours, TLC traces the complete reaction of the raw materials , extract excess ammonia gas, evaporate the solvent to obtain the crude product, add 1L deionized water and 5L ethanol, precipitate a whi...
Embodiment 3
[0045] Step 1: Add 3mol (1eq) L-histidine to 500mL tetrahydrofuran and 1L water at 0-5°C, add 9mol (3eq) sodium carbonate, stir for 60min, slowly add 6mol (2eq) 3-chloropropionyl chloride dropwise After the dropwise addition, keep it warm at 0-5°C for 8 hours, track the complete reaction of L-histidine by TLC, let it stand for 30 minutes to separate the phases, wash the tetrahydrofuran phase with 1L saturated brine, dry over anhydrous sodium sulfate, and reduce pressure below 50°C Evaporate to dryness to obtain Compound A as a yellow oil.
[0046] The second step: Add the compound A obtained in the first step into a high-pressure reactor, add 6L of 20wt% ammonia water, vacuumize and fill with ammonia gas to 0.4MPa, heat to 80°C, keep the temperature for 14 hours, TLC traces the reaction of the raw materials completely , extract excess ammonia gas, evaporate the solvent to obtain the crude product, add 200mL deionized water and 800mL ethanol, precipitate a white solid, obtain 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com