Preparation method of triethyl boron
A technology of triethyl boron and boron trifluoride diethyl ether, applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., can solve the problem of shortening the reaction time and the final yield of less than 80% %, deviation and other issues, to achieve the effect of improving sufficient mixing, changing the purification sequence, and stabilizing the reaction environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
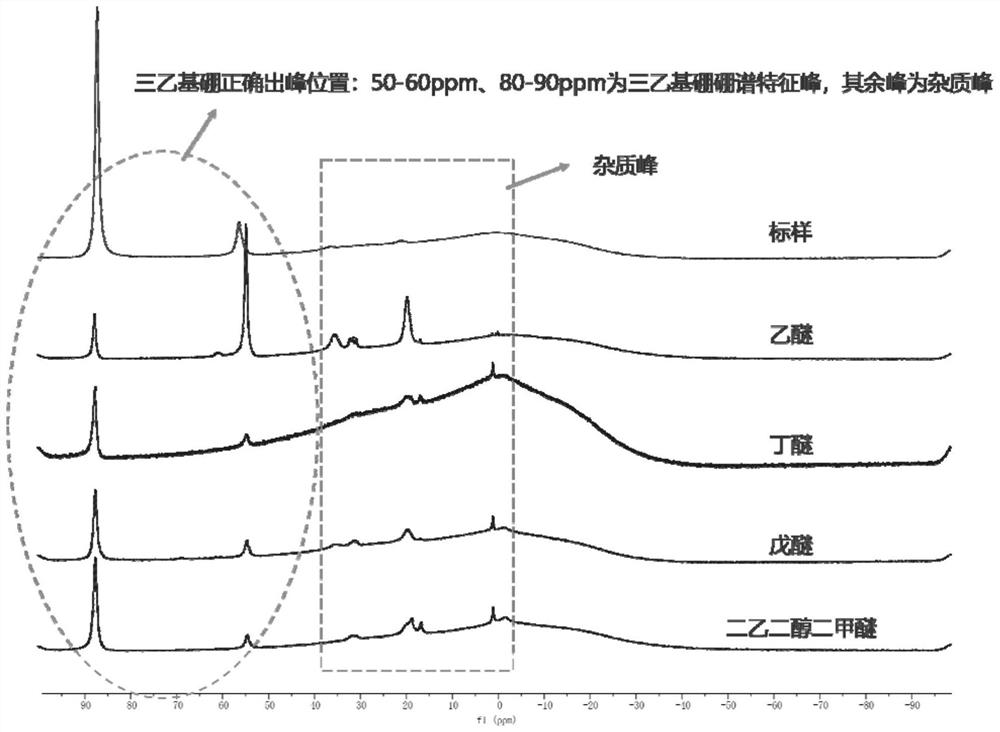
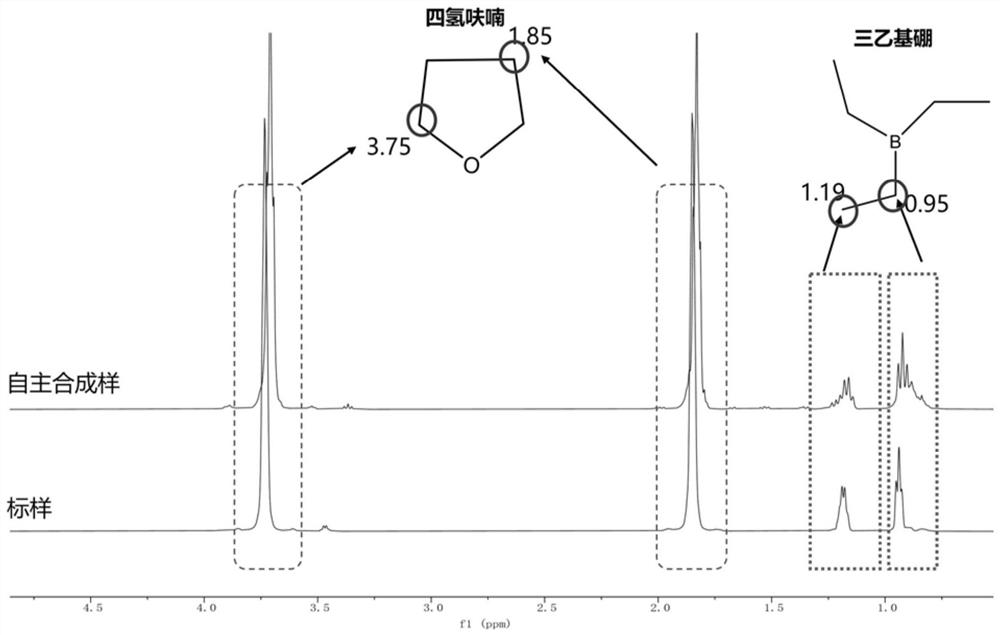
Image
Examples
Embodiment 1
[0027] With a 250mL three-necked flask as a reaction vessel, weigh magnesium powder (5.60g, 230mmol), boron trifluoride ether (9.465ml, 75mmol), anhydrous butyl ether 150ml, bromoethane (16.778ml, 225mmol). Put magnesium powder, boron trifluoride ether, 50ml of anhydrous butyl ether, 1-2 iodine tablets, and a magnetic stirrer into a three-neck bottle, seal each bottle with a glass stopper, and the remaining 100ml of anhydrous butyl ether Add the constant pressure dropping funnel, then add bromoethane (wherein the molar ratio of the substances participating in the reaction is: boron trifluoride ether: magnesium powder: bromoethane=1:3.01~3.03:3, the amount of iodine is magnesium powder 1% of mass). After taking it out, connect the reactor to the vacuum nitrogen pipeline to ensure that the entire reaction system is anhydrous and oxygen-free. At the same time, insert the ultrasonic probe, connect the sand core filter ball and the filtrate collection device. Put the reaction thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com