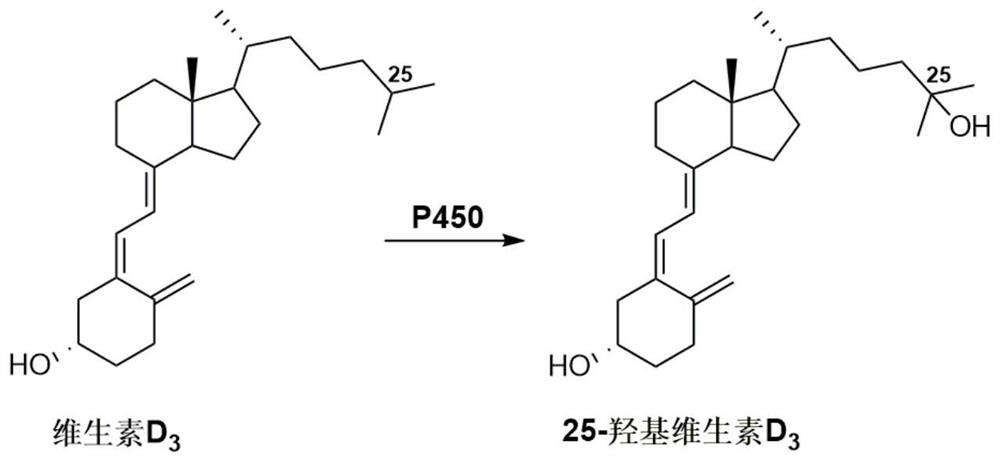
P450 enzyme mutant and application thereof
A mutant, the technology of P450BM3F87A, is applied in the field of P450 enzyme mutants that catalyze the synthesis of 25-hydroxyvitamin D3 from vitamin D3, and achieves the effects of high protein expression, broad application prospects and mature technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Construction of P450 BM3 mutant library
[0045] According to the crystal structure of P450 BM3 complexed with natural fatty acid substrate N-palmitoylglycine (PDB: 1JPZ), 11 key amino acid residues (L75, V78, F81, A82, A180) within 5 angstroms of the active pocket of the substrate were selected , L181, A184, L188, A328, A330, I263) were divided into 6 groups based on the principle of proximity, followed by A (L75, V78), B (F81, A82), C (A180, L181), D (A184, L188) , E (A328, A330), F (I263). Using P450BM3 F87A as the starting template, the mature degenerate NDT codon technology was used to carry out combined mutations of 12 different amino acids, and a mutant library with a coverage rate of >95% and a library capacity of >2000 was constructed.
[0046] The specific steps are: use the gene whose nucleotide sequence is SEQ ID NO.6 as a template, and use A-F / A-R or B-F / B-R or C-F / C-R or D-F / D-R or E-F / E-R or F-F / F-R as primers to carry out PCR amplification res...
Embodiment 2
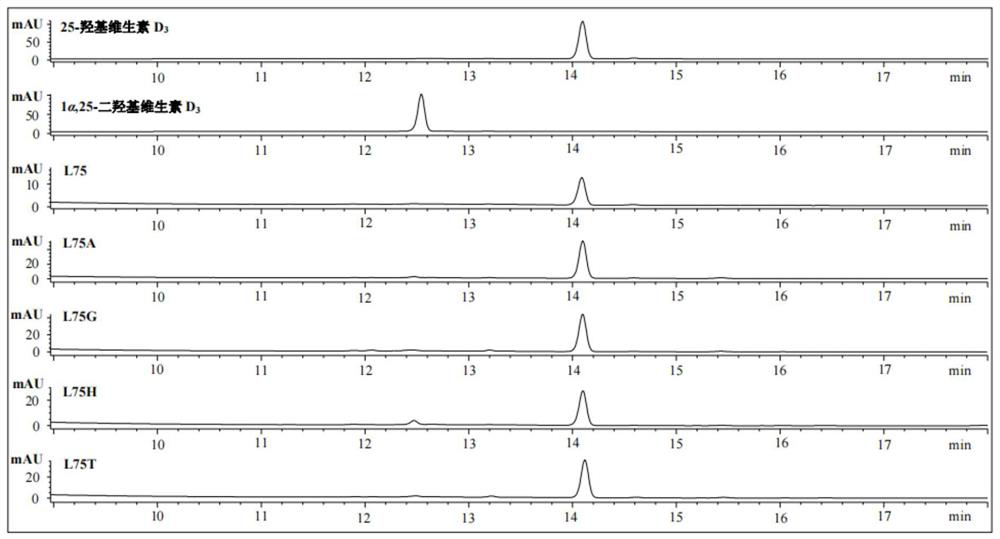
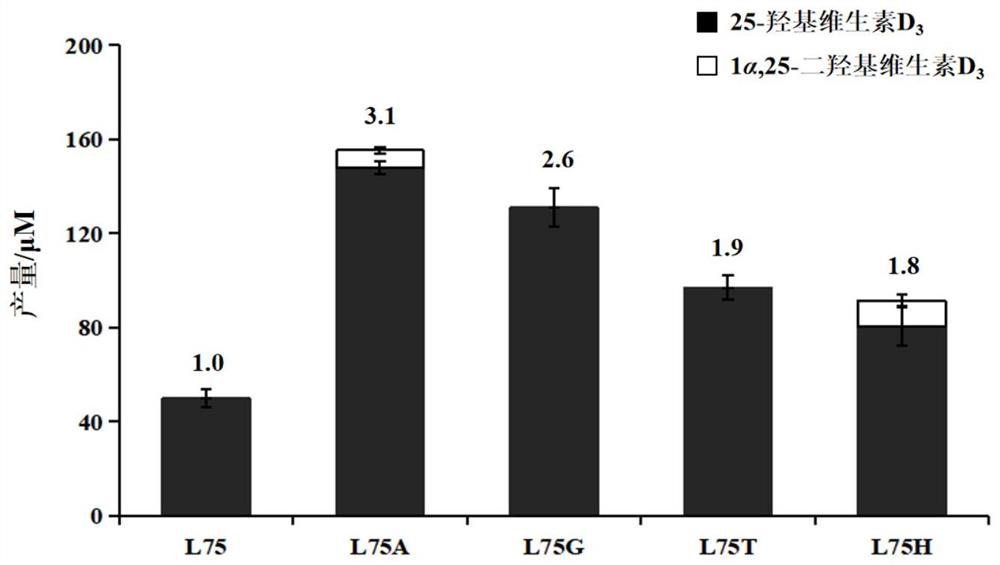
[0050] Example 2 Screening of Mutant Library and Confirmation of Highly Active Transformation Vitamin D 3 Produces 25-hydroxyvitamin D 3 mutant
[0051] Inoculate the P450 BM3 mutant bacterial liquid of the A library or B library or C library or D library or E library or F library stored in the 96 deep-well plate in Example 1 into 300 μL LB containing 50 μg / mL kanamycin respectively. Liquid medium in sterile 96 deep well plates. The culture was cultured overnight at 37°C and 220 rpm, and 40 μL of the bacterial solution from each well was transferred as a seed solution to a new sterile 96 deep-well plate containing 400 μL of TB liquid medium containing 50 μg / mL kanamycin.
[0052] After the medium was cultured at 37°C and 220rpm for a period of time (OD 600 =1.0 or so), add 0.2mM isopropyl-β-dithiogalactopyranoside (IPTG) and 0.5mM 5-aminolevulinic acid (5-ALA) to each individual well and cultured at 18° C. and 230 rpm for 24 hours to obtain mutant protein cells of library ...
Embodiment 3
[0056] Example 3 Construction of L75 position saturation mutant library
[0057] In the screening process of the mutant library in Example 2, it was found that the mutant P450 BM3 F87A / L75H at the L75 position had higher catalytic activity. Considering that the NDT degenerate codon can only derive 12 amino acid residues, this position was carried out. Saturation mutation of the point (L75) to realize the catalytic activity comparison of 20 kinds of amino acids at this point.
[0058] The mature degenerate NNK codon technology was used for saturation mutation, and the specific steps were: PCR amplification was performed with the gene whose nucleotide sequence was SEQID NO.6 as a template and L75-F / L75-R as primers (Table 2). PCR program: 5×PrimeSTARGXL Buffer 10μL, 200μM dNTPs, 0.3μM upstream and downstream primers, appropriate amount of DNA template (10-100ng), high-fidelity polymerase (PrimeSTAR GXL DNA polymerase) 2.5U, ddH 2 O was added to 50 μL; the reaction conditions we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com