Synthesis process of methylacrolein
A technology for the synthesis of methacrolein, which is applied in the direction of carbon-based compound preparation, organic compound preparation, organic compound/hydride/coordination complex catalyst, etc., and can solve the difficulty of industrial production and the yield of methacrolein It is difficult to improve the difficulty, yield and high selectivity are difficult to balance, to achieve the effect of improving selectivity and yield, saving costs, and avoiding complex reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
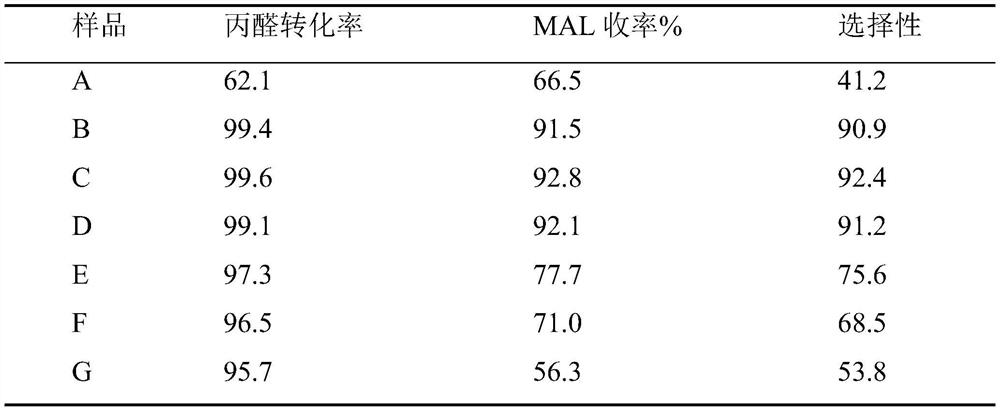
Examples
Embodiment 1
[0024] A synthesis technique of methacrolein, the specific operation steps of the synthesis technique are: firstly add 17.4g of morpholine in the reaction flask, dropwise add 11.4g of glacial acetic acid under room temperature conditions (the mol ratio of glacial acetic acid and morpholine is 0.95 g) ), add deionized water to the reaction system before the dropwise addition; after the dropwise addition, stir evenly to obtain a morpholine acetate solution, then heat up to 40 ° C, continue to stir, and then add the polymerization inhibitor nitroxyl piperidine Alcohol 1.7g, start to slowly drip the mixed solution of formaldehyde and propionaldehyde, in the mixed solution, formaldehyde 31.5g, propionaldehyde 58.0g (the mol ratio of formaldehyde and propionaldehyde is 1.05, the mol ratio of morpholine acetate solution and propionaldehyde is 0.2), after the dropwise addition, continue to react for a certain period of time, after the completion of the reaction, the reaction solution i...
Embodiment 2
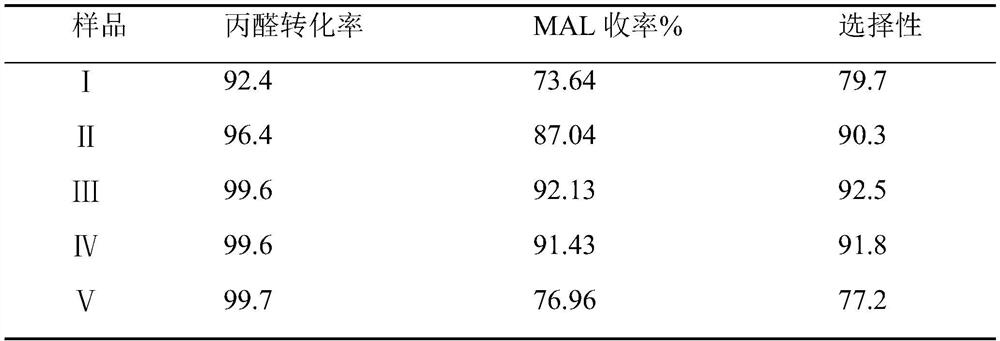
[0027] The synthesis technique of methacrolein, the concrete operation steps of its synthesis technique and the difference of embodiment 1 are the mol ratios 0.8, 0.9, 1.0, 1.1, 1.2 of formaldehyde and propionaldehyde. The aldehydes are numbered I, II, III, IV, and V. The products obtained after the reaction were sampled and detected by GC, respectively, to obtain the conversion rate of propionaldehyde and the yield and selectivity of methacrolein. The experimental data are shown in Table 1.
[0028] Table 1 embodiment 2 methacrolein product detection result
[0029]
[0030] As can be seen from the above table, the conversion rates of the propionaldehyde groups III and IV are all above 99%, and the yield and selectivity of methacrolein are also above 90%. Therefore, it can be seen that the synthesis of the technical solution of the present application The process can take into account high yield and high selectivity at the same time, and compared with the existing methacro...
Embodiment 3
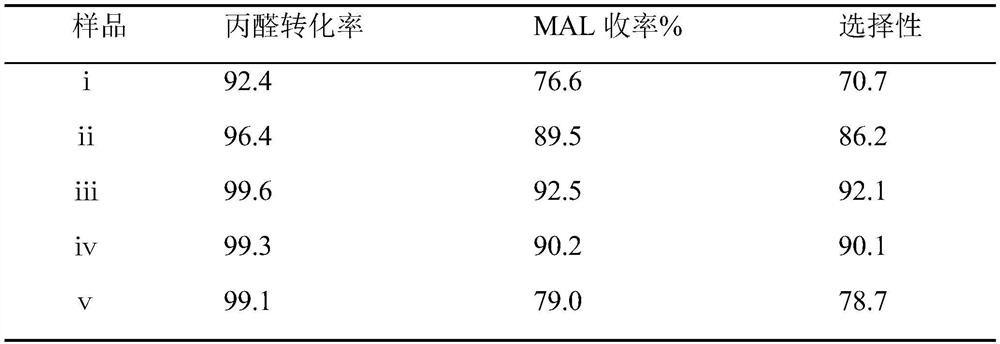
[0032] The synthesis technique of methacrolein, wherein the mol ratio of formaldehyde and propionaldehyde is 1.0, the mol ratio of acetic acid and morpholine is 0.7, 0.9, 1.1, 1.3, 1.5, and the above-mentioned different mol ratios of acetic acid and morpholine are respectively synthesized The number of methacrolein is i, ii, iii, iv, ⅴ;
[0033] Other steps are the same as in Example 1.
[0034] The product obtained after the reaction was sampled and detected by GC to obtain the conversion rate of propionaldehyde and the yield and selectivity of methacrolein. The experimental data are shown in Table 2.
[0035] The detection result of table 2 embodiment 3 methacrolein product
[0036]
[0037] It can be seen from the experimental data in the above table that with the increase of the ratio of glacial acetic acid and morpholine, the conversion rate of propionaldehyde and the yield of MAL showed a trend of first increasing and then decreasing. Comparing ⅰ-ⅴ, it can be seen t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com