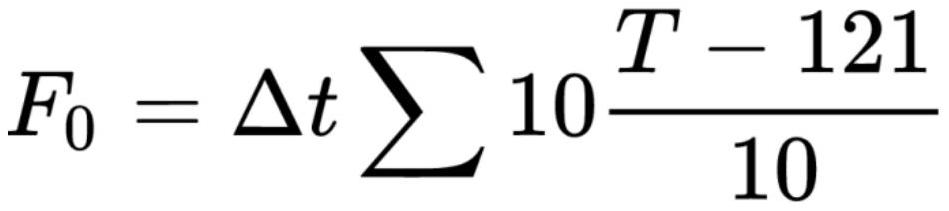Sodium potassium magnesium calcium glucose injection composition and preparation method thereof
A technology of glucose injection and injection, which is applied in the field of medicine, can solve the problems of increasing the risk of use and the increase of impurities in glucose degradation, and achieve the effects of reducing allergic reactions, reducing irritation, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of sodium potassium magnesium calcium glucose injection (250ml / bag, 21000 bags)
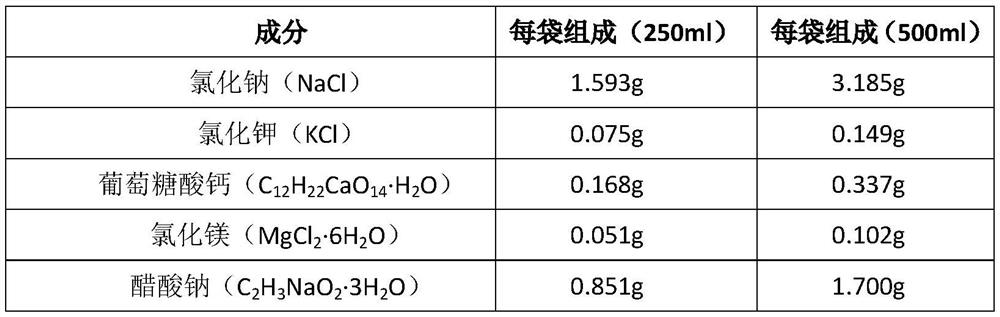
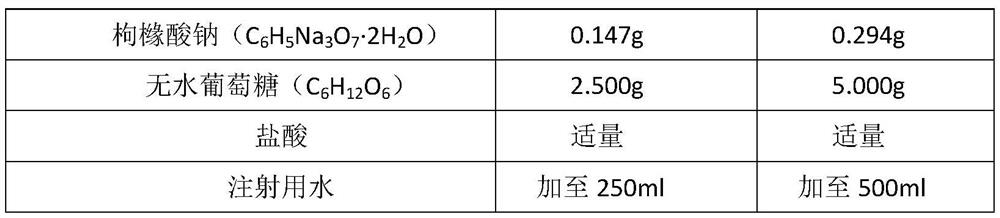
[0030] Table 1 Composition and dosage of sodium potassium magnesium calcium glucose injection (250ml / bag, 21000 bags)
[0031] The item name Each bag consists of 21,000 bags of batch prescription quantity sodium chloride 1.593g 33.453Kg potassium chloride 0.075g 1.575Kg magnesium chloride 0.051g 1.071Kg Calcium gluconate 0.168g 3.528Kg Sodium citrate 0.147g 3.087Kg Sodium acetate 0.851g 17.871Kg Glucose without anhydrous 2.500g 52.500Kg hydrochloric acid Amount Amount Water for injection Add to 250ml Added to 5250L
[0032] Preparation method:
[0033] 1) Using a stainless steel preparation tank with a stirring paddle, first add about 25% of the total weight of the water for injection, put in the prescribed amount of calcium gluconate, stir to dissolve;
[0034] 2) Add water for injection to the prepa...
Embodiment 2
[0038] Example 2: Preparation of sodium potassium magnesium calcium glucose injection (500ml / bag, 15000 bags)
[0039]Table 2 Composition and dosage of sodium potassium magnesium calcium glucose injection (500ml / bag, 15000 bags)
[0040]
[0041]
[0042] Using the same method as Example 1 to prepare sodium potassium magnesium calcium glucose injection, the difference is: the prescription amount of the two specifications there is a difference in the last digit due to rounding.
Embodiment 3
[0043] Example 3: sodium potassium magnesium calcium glucose injection pH value study
[0044] Table 3 pH filter results
[0045]
[0046] Note: "*" indicates absorbance detection value.
[0047] From the results of Table 3, it can be seen that the glucose content in the solution after sterilization is basically unchanged when the sterilization conditions are 121 °C for 15 min and 121 °C sterilization for 12 min, and the pH value is 5.0 and 5.5. When the pH value exceeds 5.5, with the increase of the pH value, the glucose content in the solution after sterilization gradually decreases, and the amount of degraded impurity fructose gradually increases.
[0048] The results of the sterilized solution under sterilization conditions of 121 °C for 8 min are as shown in Table 4.
[0049] Table 4 Results of further studies at pH
[0050]
[0051] Note: 1) "*" indicates the absorbance detection value; 2) This batch of liquid drugs is sterilized at 121 °C for 8 min.
[0052] From the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com