P-type doped material based on triaryl boron-nitrogen skeleton and preparation method and application thereof
A technology of triaryl boron nitrogen and doping materials, which is applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, compounds containing elements of Group 3/13 of the periodic table, etc., and can solve the shortage of high-efficiency P-type dopant materials , low luminous efficiency, fast aging speed and other problems, to achieve the effect of enhancing hole injection ability, improving luminous efficiency, and improving performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
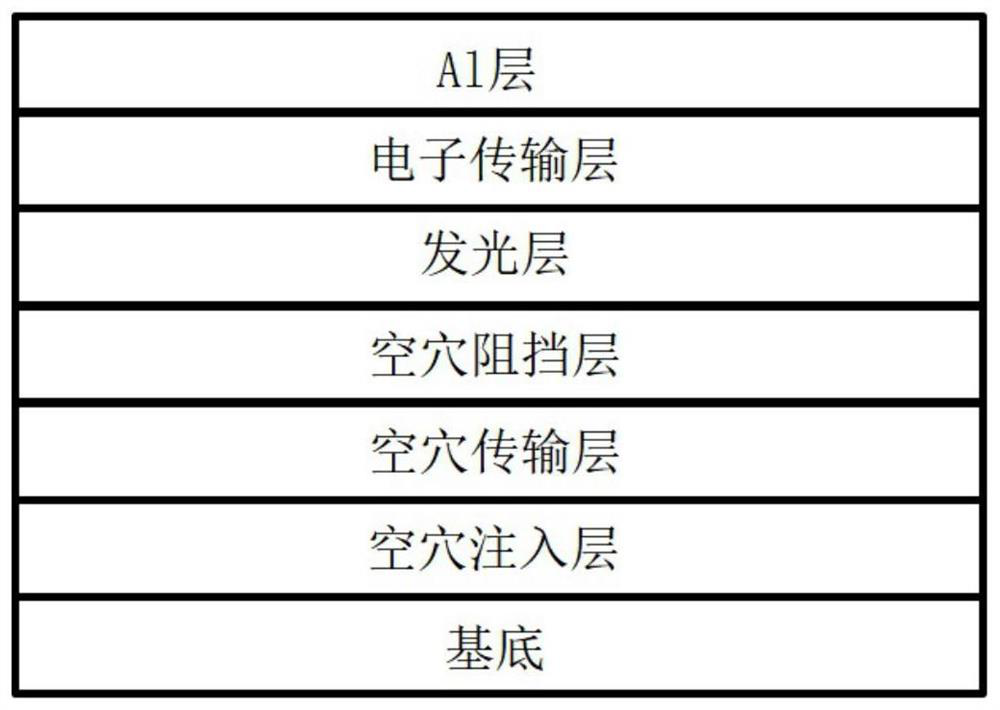
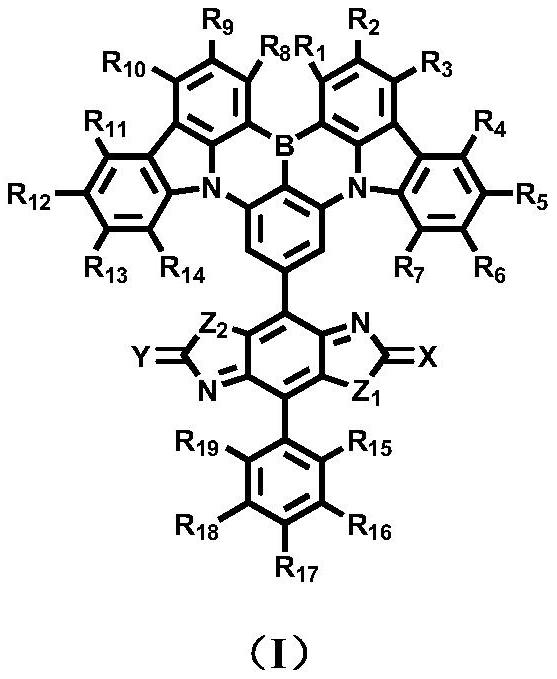
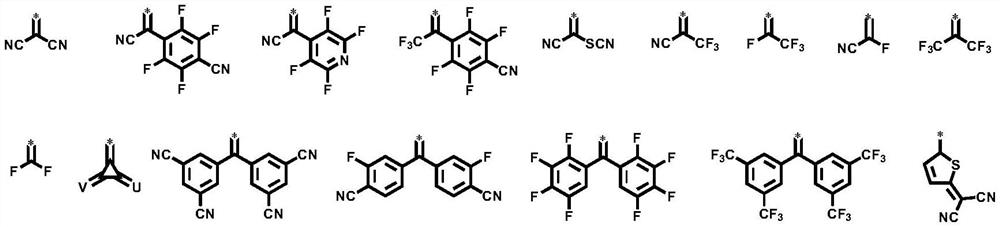
Image
Examples
Embodiment 1
[0139] Preparation of compound 2
[0140] The chemical synthesis reaction scheme is as follows:
[0141]
[0142] Synthesis of Intermediate S1: A mixture of ethyl orthoformate (22.20 g, 150 mmol), yttrium trifluoromethanesulfonate (1.30 g, 2.5 mmol) and 100 mL of dimethyl sulfoxide was heated to 60 °C in batches and (about 0.50g each time, added within 10min) add 3,6-diamino-2,5-dibromo-1,4-hydroquinone (14.90g, 50mmol), stir overnight at 60°C, then cool to room temperature And dilute with water. The resulting precipitate was collected by filtration and washed with distilled water and cold ethanol, yield 75%. Molecular formula C 8 h 2 Br 2 N 2 O 2 , ESI(H + ): 316.8566.
[0143] Synthesis of Intermediate 2-1: Intermediate S1 (6.36g, 20mmol), p-fluorophenylboronic acid (3.08g, 22mmol), 300mL toluene and 80mL water mixture, add catalyst tetrakis (triphenylphosphine) palladium (0.12g , 0.1 mmol) and potassium carbonate (4.16 g, 30 mmol). The system was heated to ref...
Embodiment 2
[0151] Preparation of compound 12
[0152] The chemical synthesis reaction scheme is as follows:
[0153]
[0154] Synthesis of Intermediate 12-1: According to the synthesis of Intermediate 2-1, an equimolar amount of 2,4,6-trifluorophenylboronic acid was substituted for p-fluorophenylboronic acid, and the yield was 70%. Molecular formula C 14 H 4 BrF 3 N 2 O 2 , ESI(H + ): 368.9482.
[0155] Synthesis of Intermediate 12-2: According to the synthesis of Intermediate 2-2, intermediate 2-1 was replaced with an equimolar amount of Intermediate 12-1, and the yield was 82%. Molecular formula C 14 H 6 BF 3 N 2 O 4 , ESI(H + ): 335.0449.
[0156] Synthesis of Intermediate 12-3: According to the synthesis of Intermediate 2-4, intermediate 2-2 was replaced with an equimolar amount of Intermediate 12-2, and the yield was 68%. Molecular formula C 44 H 18 ClF 7 N 4 O 2 , MALDI-TOF (m / z): 802.23.
[0157] Synthesis of intermediate 12-4: According to the synthesis of...
Embodiment 3
[0161] Preparation of Compound 13
[0162] The chemical synthesis reaction scheme is as follows:
[0163]
[0164] Synthesis of Intermediate 13-1: According to the synthesis of Intermediate 2-1, an equimolar amount of 3,4,5-trifluorophenylboronic acid was substituted for p-fluorophenylboronic acid, and the yield was 68%. Molecular formula C 14 H 4 BrF 3 N 2 O 2 , ESI(H + ): 368.9484.
[0165] Synthesis of Intermediate 13-2: According to the synthesis of Intermediate 2-2, intermediate 2-1 was replaced with an equimolar amount of Intermediate 13-1, and the yield was 84%. Molecular formula C 14 H 6 BF 3 N 2 O 4 , ESI(H + ): 335.0452.
[0166] Synthesis of Intermediate 13-3: According to the synthesis of Intermediate 2-4, intermediate 2-2 was replaced with an equimolar amount of Intermediate 13-2, and the yield was 70%. Molecular formula C 44 H 18 ClF 7 N 4 O 2 , MALDI-TOF (m / z): 802.16.
[0167] Synthesis of Intermediate 13-4: According to the synthesis of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com