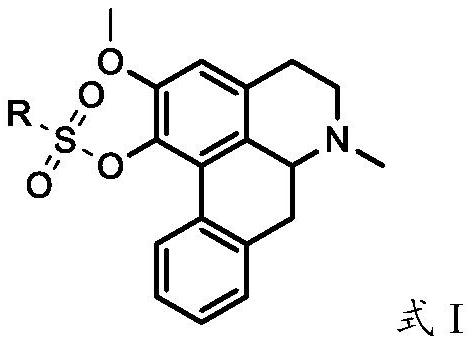
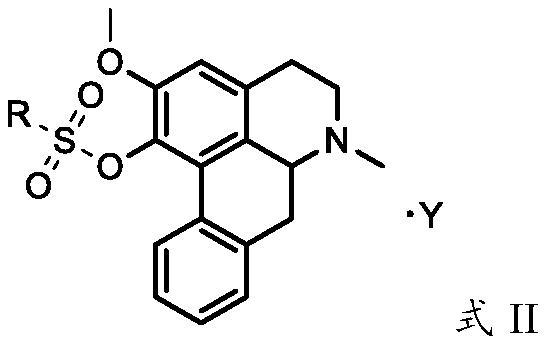
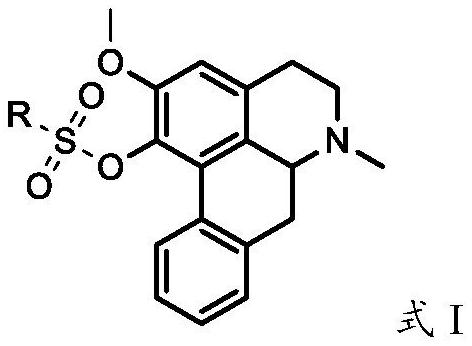
Nuciferine derivative and application of nuciferine derivative in preparation of uric acid reducing medicine
A technology of nuciferine and medicine, applied in the field of nuciferine derivatives and its application in the preparation of uric acid-lowering drugs, can solve problems such as mental confusion, hearing loss, coma, etc., and achieve excellent activity, high uric acid activity, The effect of strong uric acid activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0327] Example 1 Synthesis of 1-Ethyl sulfone
[0328]Accurately weigh 2.81 g (10 mmol) of 1-O-desmethyl lotus leaf base, dissolve it in 50 mL of chloroform, place it in a nitrogen-filled three-necked flask, add it and dissolve it in 120 mL of piperidine under magnetic stirring, and control the temperature to 0-5 ℃ mixed for 1.0h, added 2.58g (about 12mmol) of ethylsulfonyl chloride dissolved in 100mL of chloroform, washed with 100mL of deionized water for 3-5 times after the reaction, and concentrated the organic phase to about 10mL by rotary evaporation. Alumina column separation and purification (eluent v / v: chloroform / methanol=150 / 1-100 / 1), collecting product fractions, steaming the solvent under reduced pressure to obtain the product, TLC tracking the reaction and the separation and purification process of the product, 2.26g of light green powder product was obtained, the melting point of the product was 87.0-88.7°C, 1 H NMR, 13 By C NMR and HR-MS analysis, it was det...
Embodiment 2
[0329] Example 2 Synthesis of 1-n-propanesulfonate-based susceptin
[0330] Accurately weigh 2.81 g (10 mmol) of 1-O-desmethyl succinate, dissolve it in 100 mL of dichloromethane and place it in a nitrogen-filled three-necked flask, add 5.06 g of triethylamine dissolved in 50 mL of dichloromethane under magnetic stirring (about 50 mmol), control the temperature to 0-5 °C and mix for 2.0 h, add 1.71 g (about 12 mmol) of n-propylsulfonyl chloride dissolved in 100 mL of dichloromethane, and wash with 100 mL of deionized water for 3-5 times after the reaction. The organic phase was concentrated to about 10 mL by rotary evaporation, separated and purified by a neutral alumina column (eluent v / v: dichloromethane / methanol=150 / 1-100 / 1), the product fractions were collected, and the solvent was evaporated under reduced pressure to obtain product, TLC followed the reaction and the separation and purification process of the product to obtain 2.89g of light yellow oily product. 1 H NM...
Embodiment 3
[0331] Example 3 Synthesis of 1-n-butanesulfonate susceptin
[0332] Accurately weigh 2.81 g (10 mmol) of 1-O-desmethyl succinate, dissolve it in 120 mL of tetrahydrofuran and place it in a nitrogen-filled three-necked flask, add γ-Al under mechanical stirring 2 O 3 -Na 5.60g, after mixing for 1.0h, add 3.13g (about 20mmol) of 1-n-butylsulfonyl chloride dissolved in 100mL of tetrahydrofuran, control the temperature to -20~-5℃ and react for about 10h, then add 3g of activated carbon after the reaction Mixed for 2 h, filtered, the filter cake was washed 2-3 times with 100 mL of tetrahydrofuran, the filtrate was collected, concentrated and evaporated to about 20 mL, crystallized at room temperature overnight, TLC followed the separation and purification process of the reaction and the product, filtered, and the solid was dried at 60 ° C for 4 h, 1.86g of pale green powder was obtained, the melting point of the product was 90.2-91.7°C, 1 H NMR, 13 By C NMR and HR-MS analysis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com