Preparation method of 5-bromobenzofuranone
A technology of furanone and bromoacetophenone, which is applied in the field of preparation of 5-bromobenzofuranone, can solve the problems of low yield, low yield, low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
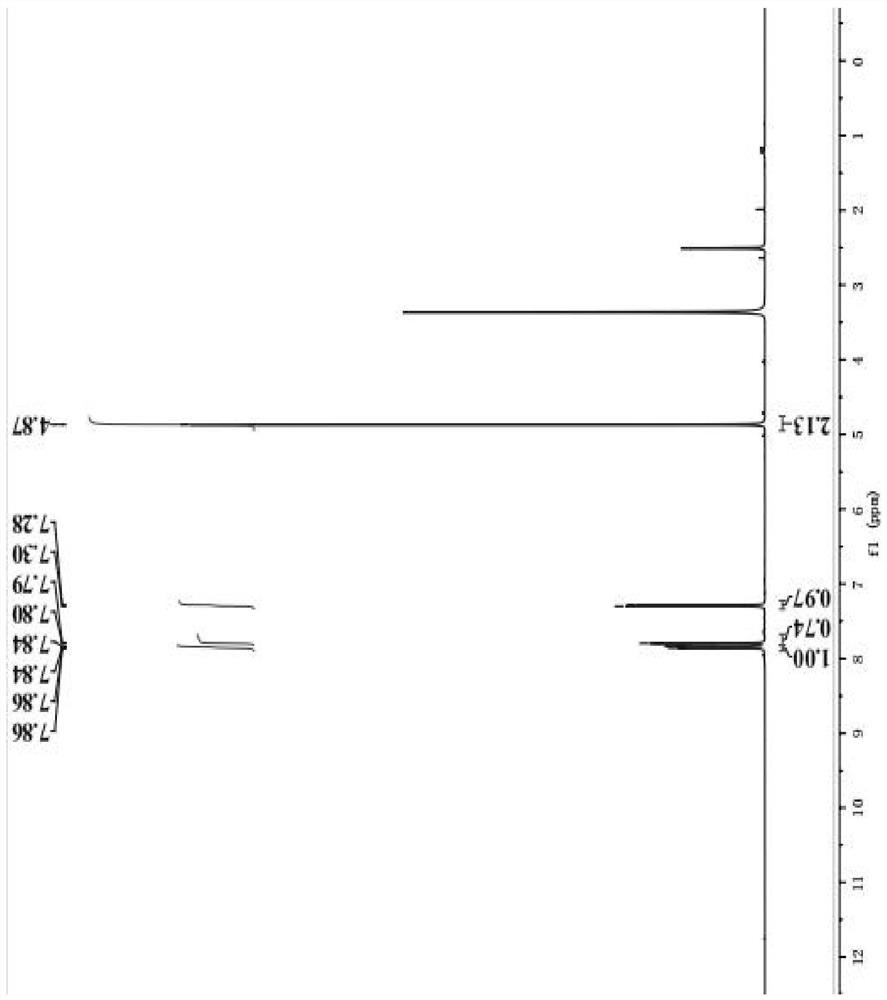
[0024] The invention provides a preparation method of 5-bromobenzofuranone, comprising the following steps:
[0025] Mix 2-hydroxy-5-bromoacetophenone, bromination reagent, catalyst and first organic solvent, and carry out bromination reaction to obtain 2-bromo-1-(5-bromo-2-hydroxyphenyl)-ethanone ;
[0026] The 2-bromo-1-(5-bromo-2-hydroxyphenyl)-ethanone, the organic base and the second organic solvent are mixed to carry out a cyclization reaction to obtain 5-bromobenzofuranone.
[0027] Unless otherwise specified, the preparation raw materials used in the present invention are all commercially available.
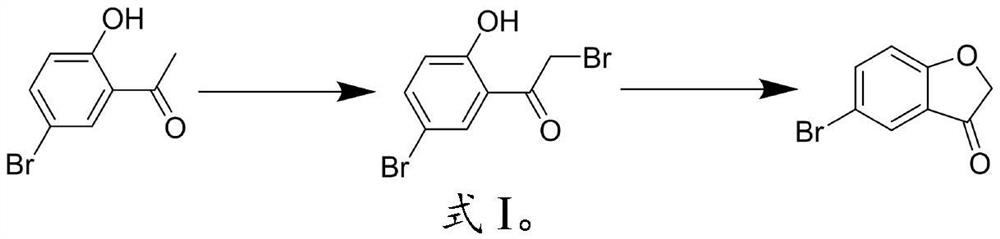
[0028] In the present invention, the synthetic route of described 5-bromobenzofuranone is shown in formula 1:
[0029]
[0030] In the present invention, 2-hydroxy-5-bromoacetophenone, a bromination reagent, a catalyst and a first organic solvent are mixed, and a bromination reaction is carried out to obtain 2-bromo-1-(5-bromo-2-hydroxyphenyl)- ethyl ketone. In the...
Embodiment 1
[0038] Add 10g 2-hydroxy-5-bromoacetophenone in the reactor, dissolve it in the solvent that 50mL ethyl acetate and 50mL 1,2-dichloroethane form, then add 7.43g liquid bromine, 0.8g successively copper bromide, the temperature of the reaction kettle was heated to 100 ° C and stirred for 12 h, and the reaction progress was monitored in the gas phase. After the reaction, the reaction kettle was lowered to room temperature, and the solid impurities in the reaction system were removed by filtration to obtain a filtrate, and the filtrate was rotary-evaporated at 40 ° C until the solvent was evaporated to dryness to obtain 2-bromo-1-(5-bromo-2- Hydroxyphenyl)-ethanone crude product. Then, 20 mL of water was added to the crude product of 2-bromo-1-(5-bromo-2-hydroxyphenyl)-ethanone to redissolve it, 60 mL of dichloromethane was added for extraction, and the layers were separated to obtain a dichloromethane phase. The chloromethane phase was washed with 60 mL of brine, then the dichl...
Embodiment 2
[0041] Add 20g of 2-hydroxy-5-bromoacetophenone to the reactor, dissolve it in the solvent composed of 100mL of ethyl acetate and 100mL of chloroform, then add 14.86g of hydrobromic acid and 1.5g of iron powder successively, The temperature of the reaction kettle was heated to 80°C and the reaction was stirred for 15h, and the reaction progress was monitored in the gas phase. After the reaction, the reaction kettle was lowered to room temperature, and the solid impurities in the reaction system were removed by filtration to obtain a filtrate. The filtrate was rotary-evaporated at 35°C until the solvent was evaporated to dryness to obtain 2-bromo-1-(5-bromo-2- Hydroxyphenyl)-ethanone crude product. Then, 40 mL of water was added to the crude product of 2-bromo-1-(5-bromo-2-hydroxyphenyl)-ethanone to redissolve it, and 120 mL of chloroform was added for extraction, and the layers were separated to obtain a chloroform phase. The chloromethane phase was washed with 120 mL of brin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com