Indazole cyclotriazole compound as well as preparation method and application thereof
A technology of indazole-linked triazoles and compounds, which is applied in the field of inhibitor preparation and can solve problems such as adverse reactions and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
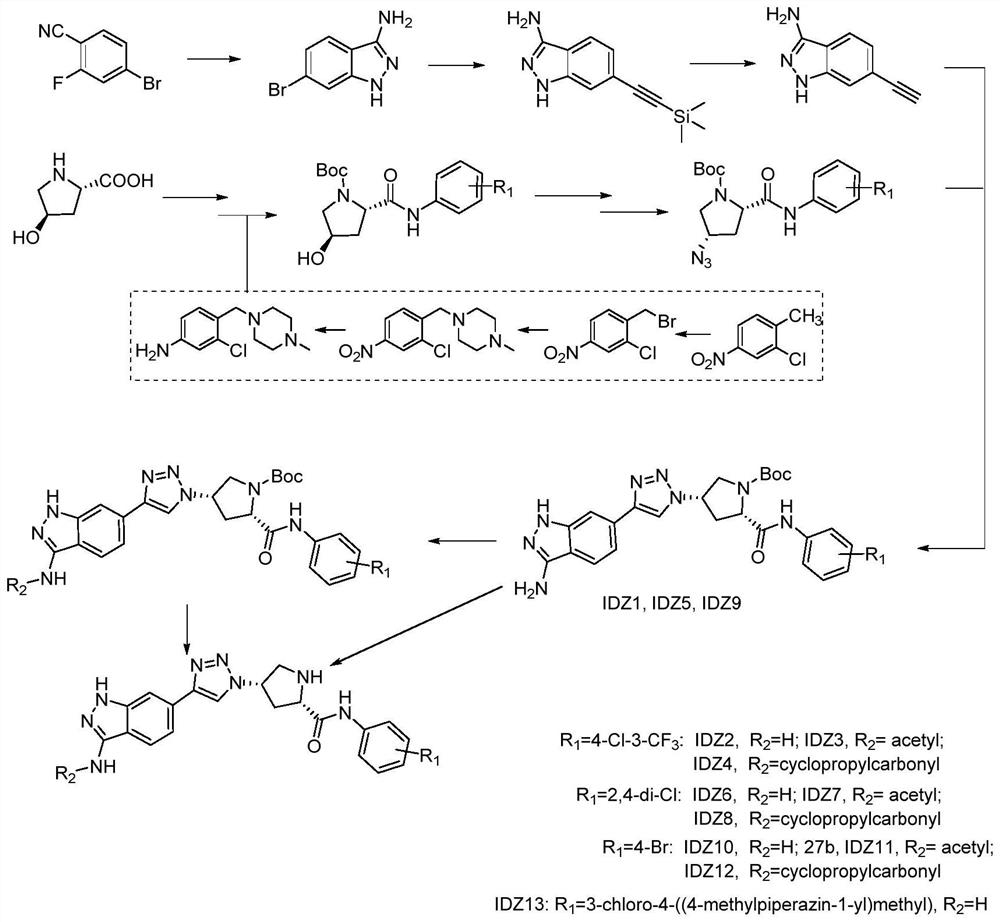
[0045] A preparation method of the above-mentioned indazole-linked triazole compound, comprising the following steps:
[0046] 1) 4-bromo-2-fluorobenzonitrile reacts with hydrazine monohydrate to prepare 6-bromo-1H-indazol-3-amine;
[0047] 2) Under nitrogen protection, 6-bromo-1H-indazol-3-amine reacts with trimethylsilyl acetylene to prepare 6-((trimethylsilyl)ethynyl)-1H-indazol-3-amine ;
[0048]3) Use tetrabutylammonium fluoride trihydrate to remove trimethylsilyl from 6-((trimethylsilyl)ethynyl)-1H-indazol-3-amine to obtain 6-ethynyl-1H- indazol-3-amine;
[0049] 4) substitution reaction of L-hydroxyproline and di-tert-butyl dicarbonate in ice-water bath to prepare L-hydroxyproline (Boc-L-hydroxyproline) protected by tert-butoxycarbonyl;
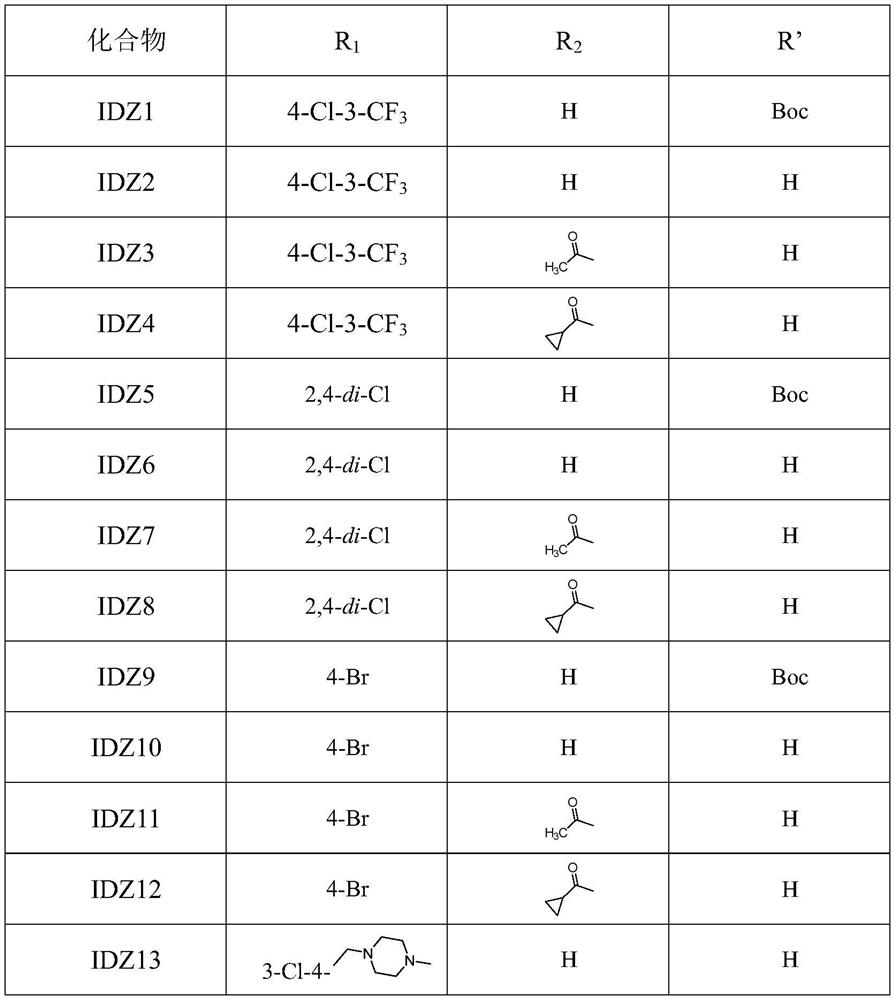
[0050] 5) Boc-L-hydroxyproline is condensed with a substituted aniline compound to form an ammoniated Boc-L-hydroxyproline compound; the substituted aniline compound is 5-amino-2-chlorotrifluoromethane benzene, 2,4-dichloroaniline,...
Embodiment 1
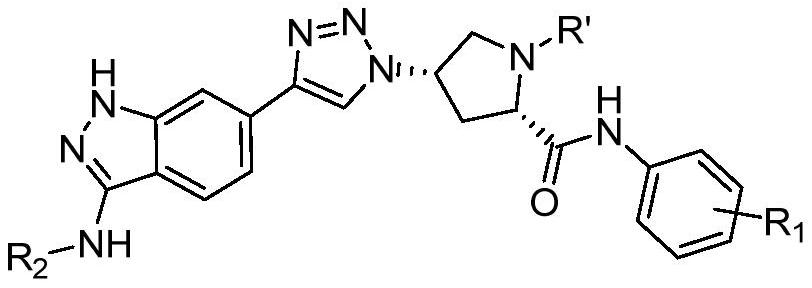
[0069] An indazole ring-linked triazole compound, R 1 4-Cl-3-CF 3 , R 2 For H, when R' is Boc or H, the preparation method is as follows:
[0070] 1) Synthesis of 6-bromo-1H-indazol-3-amine: Dissolve 4-bromo-2-fluorobenzonitrile (5.0 g, 25.1 mmol) in n-butanol (20 mL), then add hydrazine monohydrate (1.0 mL, 50.3 mmol). The reaction mixture was heated to reflux for 4 h, cooled to room temperature, filtered, washed with n-hexane and dried to obtain 4.77 g of a white solid with a yield of 85%.
[0071] 2) Synthesis of 6-((trimethylsilyl)ethynyl)-1H-indazol-3-amine: 6-bromo-1H-indazol-3-amine (3.42g, 16.12mmol), CuI ( 10%, 0.31g, 1.61mmol), and Pd (PPh 3 ) 4 (10%, 1.86 g, 1.61 mmol) was added to a 100 mL two-necked round bottom flask with condenser and magnetic stirring. The vessel was then sealed with a rubber stopper, evacuated and backfilled with nitrogen (3 times). Using triethylamine (30 mL) as base and solvent, inject with syringe. After 5 min at room temperature, ...
Embodiment 2
[0080] An indazole ring-linked triazole compound, R 1 4-Cl-3-CF 3 , R 2 When being an acetyl group, the preparation method is as follows:
[0081] Steps 1) to 8) are the same as in Example 1, to obtain (2S,4S)-4-(4-(3-amino-1H-indazol-6-yl)-1H-1,2,3-triazole -1-yl)-2-((4-chloro-3-(trifluoromethyl)phenyl)carbamoyl)pyrrolidine-1-carboxylic acid tert-butyl ester.
[0082] 9) (2S,4S)-4-(4-(3-acetylamino-1H-indazol-6-yl)-1H-1,2,3-triazol-1-yl)-N-(4 - Preparation of chloro-3-(trifluoromethyl)phenyl)pyrrolidine-2-carboxamide (IDZ3): compound (2S,4S)-4-(4-(3-amino-1H-indazole-6) -yl)-1H-1,2,3-triazol-1-yl)-2-((4-chloro-3-(trifluoromethyl)phenyl)carbamoyl)pyrrolidine-1-carboxylate Tert-butyl acid (0.30 g, 0.51 mmol) was dissolved in 30 mL of anhydrous dichloromethane and triethylamine (0.37 mL, 2.7 mmol) was added. The solution was stirred at 0° C. for 30 min, and acetyl chloride (43 μL, 0.61 mmol) in dichloromethane (2 mL) was added dropwise. The solution was reacted at room te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com