Preparation method and application of bimetallic co-doped nickel phosphide nanosheet
A technology of co-doping and nickel phosphide, applied in the field of material chemistry, can solve the problems of reducing catalysts, health and environmental hazards, and achieve the effects of accelerated electron transfer, large surface area, and good electrochemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
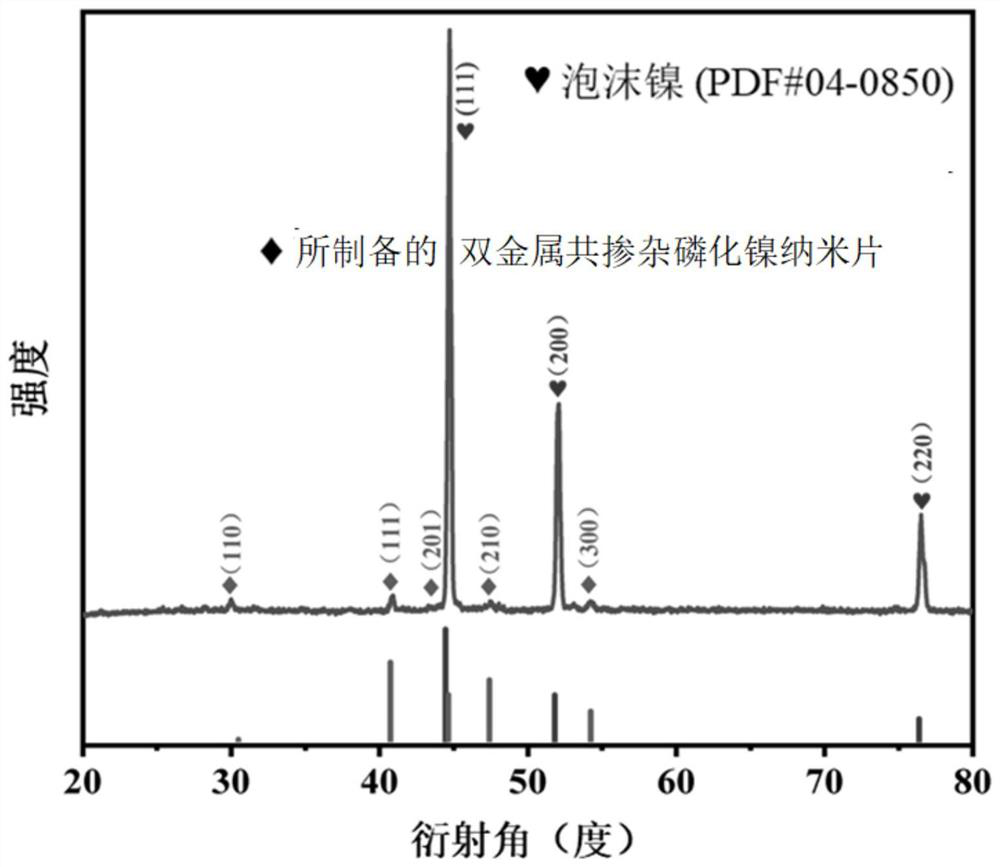
[0018] Weigh 2.0 mmol (0.3 g) of ferrous sulfate (FeSO 4 ·4H 2 O), 0.5mmol (0.098g) manganese chloride (MnCl) 2 ·4H 2 O), 2.5mmol (0.72g) nickel nitrate (Ni(NO) 3 ) 2 ·6H 2 O), 5.0 mmol (0.185 g) ammonium fluoride (NH 4 F), 10mmol (0.6g) urea (CH 4 N 2 0) was added to 35 mL of deionized water, then sealed and stirred for 1 h to obtain a clear solution; a piece of foamed nickel (2×4 cm) and the above solution were placed in a reaction kettle with a polytetrafluoroethylene lining, and at a high temperature of 160 ° C The reaction was carried out for 8 hours, then cooled to room temperature, and the nickel foam was taken out, washed with deionized water and ethanol successively, and then put into a constant temperature blast oven for drying treatment to obtain the dried nickel foam; the dried nickel foam and sodium hypophosphite were (mass ratio 1:10) was placed in a tube furnace, sintered and annealed at 350 °C for 2 hours in an argon atmosphere to obtain manganese and i...
Embodiment 2
[0020] Weigh 1.5mmol (0.225g) of ferrous sulfate (FeSO 4 ·4H 2 O), 1.0mmol (0.196g) manganese chloride (MnCl) 2 ·4H 2 O), 2.0mmol (0.576g) nickel nitrate (Ni(NO) 3 ) 2 ·6H 2 O), 5.0 mmol (0.185 g) ammonium fluoride (NH 4 F), 10mmol (0.6g) urea (CH 4 N 20) was added to 35 mL of deionized water, then sealed and stirred for 1 h to obtain a clear solution; a piece of nickel foam (2 × 4 cm) and the above solution were placed in a reaction kettle with a Teflon lining, and at a high temperature of 160 ° C React for 8h, cool to room temperature, take out the nickel foam, wash with deionized water and ethanol successively, put it into a constant temperature blast oven for drying treatment, and obtain the nickel foam after drying; the nickel foam after drying and sodium hypophosphite ( The mass ratio of 1:10) was placed in a tube furnace, and sintered and annealed at 350 °C for 2 hours in an argon atmosphere to obtain manganese and iron co-doped nickel phosphide nanosheets, that...
Embodiment 3
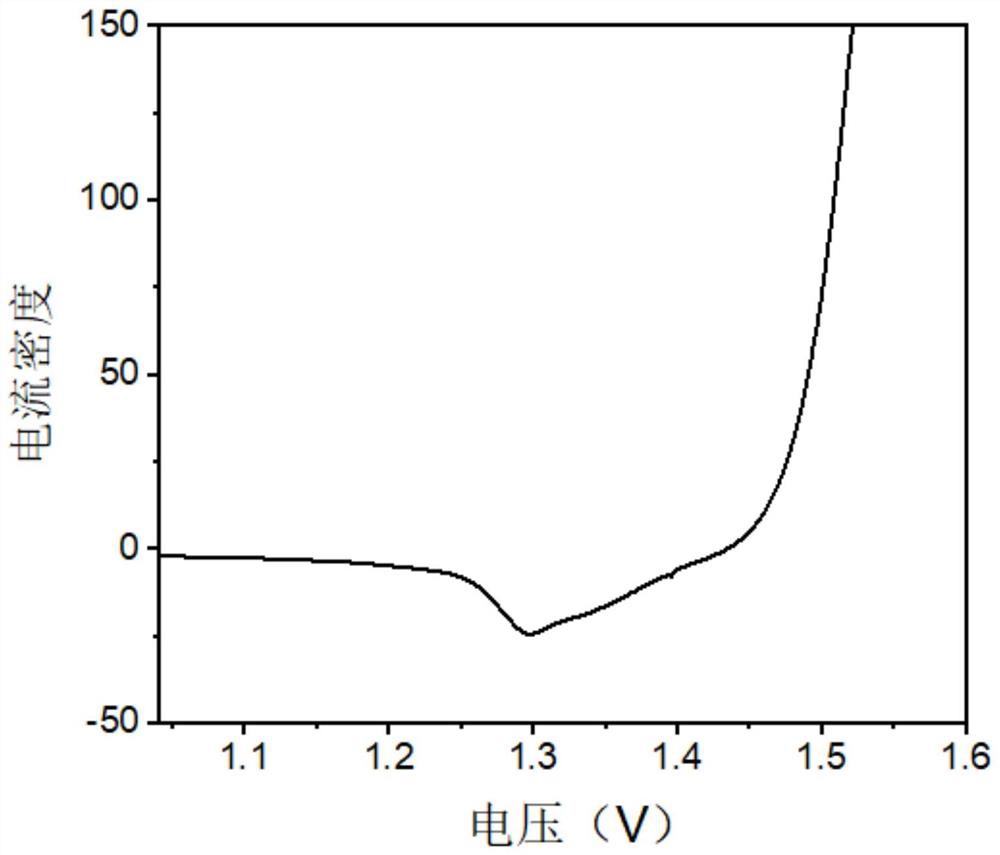
[0022] Weigh 1.0 mmol (0.15 g) of ferrous sulfate (FeSO 4 ·4H 2 O), 1.5mmol (0.294g) manganese chloride (MnCl) 2 ·4H 2 O), 2.0mmol (0.576g) nickel nitrate (Ni(NO) 3 ) 2 ·6H 2 O), 5.0 mmol (0.185 g) ammonium fluoride (NH 4 F), 10mmol (0.6g) urea (CH 4 N 2 0) was added to 35 mL of deionized water, then sealed and stirred for 1 h to obtain a clear solution; a piece of nickel foam (2 × 4 cm) and the above solution were placed in a reaction kettle with a Teflon lining, and at a high temperature of 160 ° C React for 8h, cool to room temperature, take out the nickel foam, wash with deionized water and ethanol successively, put it into a constant temperature blast oven for drying treatment, and obtain the nickel foam after drying; the nickel foam after drying and sodium hypophosphite ( The mass ratio of 1:10) was placed in a tube furnace, and burned at 350 ° C for 2 hours in an argon atmosphere to obtain a bimetallic co-doped nickel phosphide nanosheet; the obtained bimetallic...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap