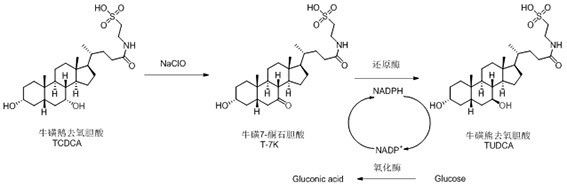
Method for preparing tauroursodeoxycholic acid by two-step method
A technology for tauroursodeoxycholic acid and tauroursodeoxycholic acid is applied in the field of preparing tauroursodeoxycholic acid by a two-step method, which can solve the problems of many reaction steps, high price, complicated preparation and the like, and achieves the The effect of short reaction time, saving raw material cost and simplifying process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Chemical oxidation of sodium hypochlorite by TCDCA to generate taurine 7-ketolithocholic acid (T-7K)
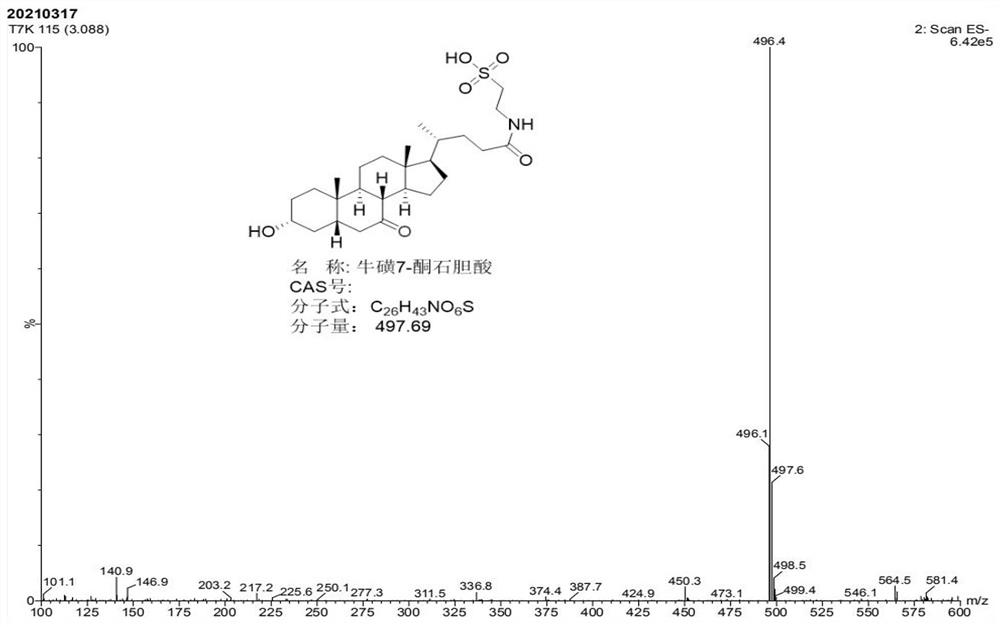
[0065] Take 300g of TCDCA (98%) and add 300mL of methanol, after dissolving, add 100g of phosphoric acid, dropwise add 300mL of sodium hypochlorite aqueous solution (available chlorine 12%), and conduct the reaction under temperature control, and the reaction process temperature does not exceed 5 °C. After the dropwise addition, the TCDCA residue was detected by liquid phase, and the TCDCA residue was determined by liquid chromatography. The TCDCA residue was 0.43%, less than 1.0%. The reaction was completed. After adding 6.0 g of anhydrous sodium sulfite and stirring for 30 min, slowly add 300 mL of water dropwise, filter and place it in. Dry in a vacuum drying oven at 75°C for 10h to obtain the product T-7K, weigh 292g, and determine the purity of 95.76%.
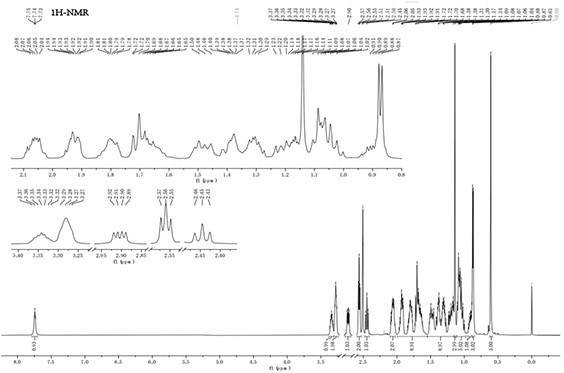
[0066] figure 2 is the MS spectrum of taurine 7-ketolithocholic acid (T-7K); image 3 is the 1H-NM...
Embodiment 2
[0067] Example 2 Preparation and expression of tauroursodeoxycholidase-reducing engineered bacteria.
[0068] 1. Whole-gene synthesis of codon-optimized 7β-HSDH gene and GDH gene
[0069] 1.7β-HSDH and GDH genes are 7β-hydroxysteroid dehydrogenase and glucose dehydrogenase, respectively
[0070] 2.7β-HSDH is derived from: Clostridium absonum (GenBank accession number: JN191345.1)
[0071] GDH source: Bacillus subtilis (GenBank accession number NC-000964)
[0072] 3. The above sequence was entrusted to Sangon Bio (Shanghai) Co., Ltd. to optimize the gene sequence according to the codon preference of Escherichia coli, and to add EcoRⅤ and XhoI enzymes to the 5' and 3' ends of the 7β-HSDH gene sequence, respectively. The cleavage site was added to the 5' and 3' ends of the GDH gene sequence, respectively, with EcoRI and HindIII restriction sites, and the whole gene was synthesized. The gene sequence of the obtained codon-optimized 7β-HSDH is shown in SEQ ID NO.1, and the gene ...
Embodiment 3
[0136] Example 3 Whole cell enzyme-catalyzed preparation of tauroursodeoxycholic acid.
[0137] Take 250 g of T-7k obtained in Example 1, add 1150 mL of purified water, add 137.5 g of dextrose monohydrate, stir well, and after the temperature reaches 25 ° C, add 50 g of the bacterial cells prepared in Example 2 (mixed with 100 mL of purified water). After suspending evenly), add NADP + After 0.25g, the reaction was started, the rotation speed was 400rpm, and the reaction pH was adjusted between 6.5 and 8.0 with 1M sodium hydroxide during the reaction. The reaction process was monitored by TLC plate, and samples were taken every 2 hours for monitoring. After 8 hours of reaction, liquid chromatography was used to determine that the residual T-7k was 0.43%, less than 1.0%. The reaction was completed, and the reaction solution of tauroursodeoxycholic acid was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com