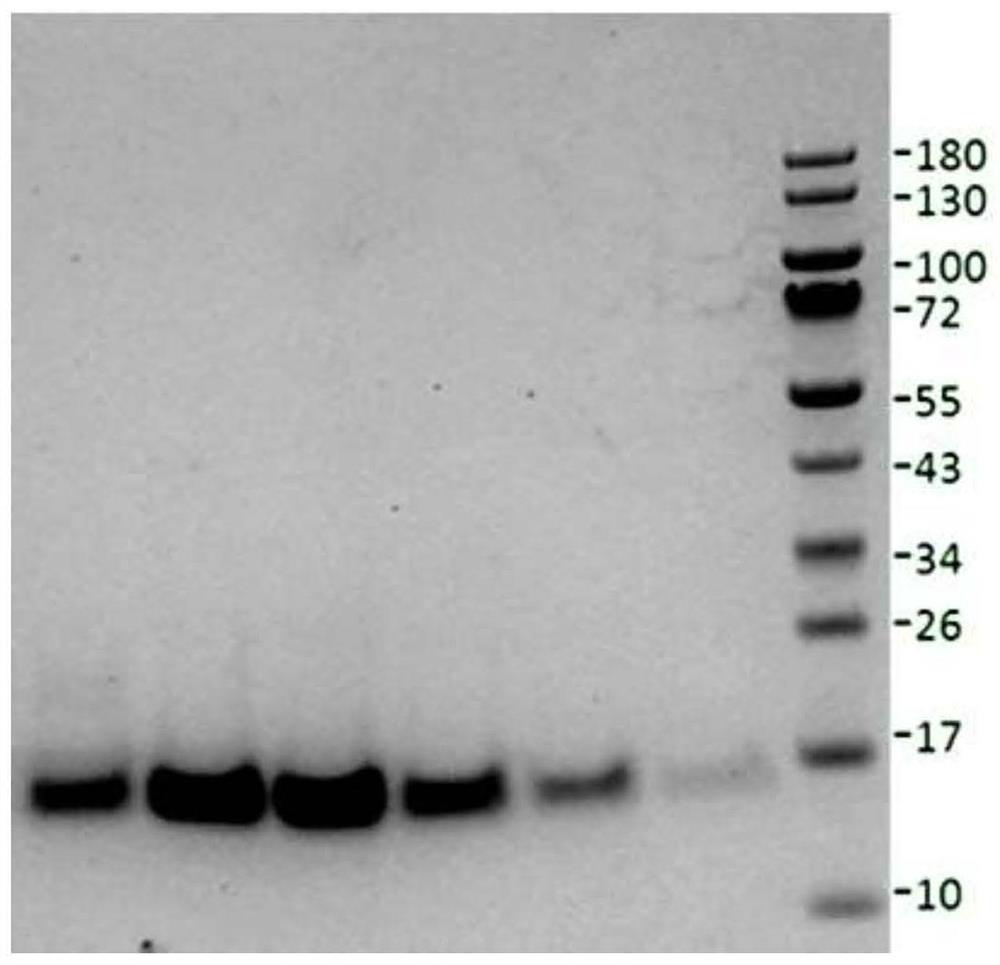
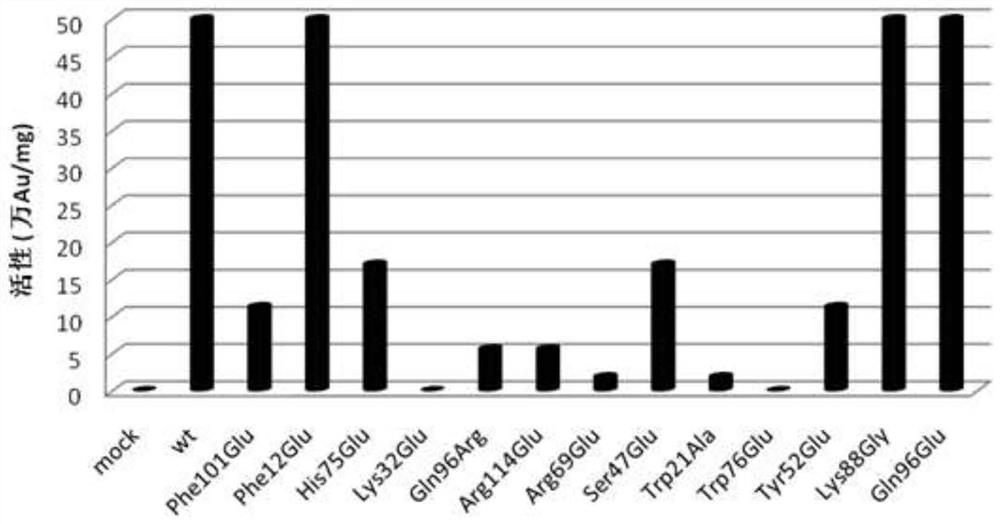
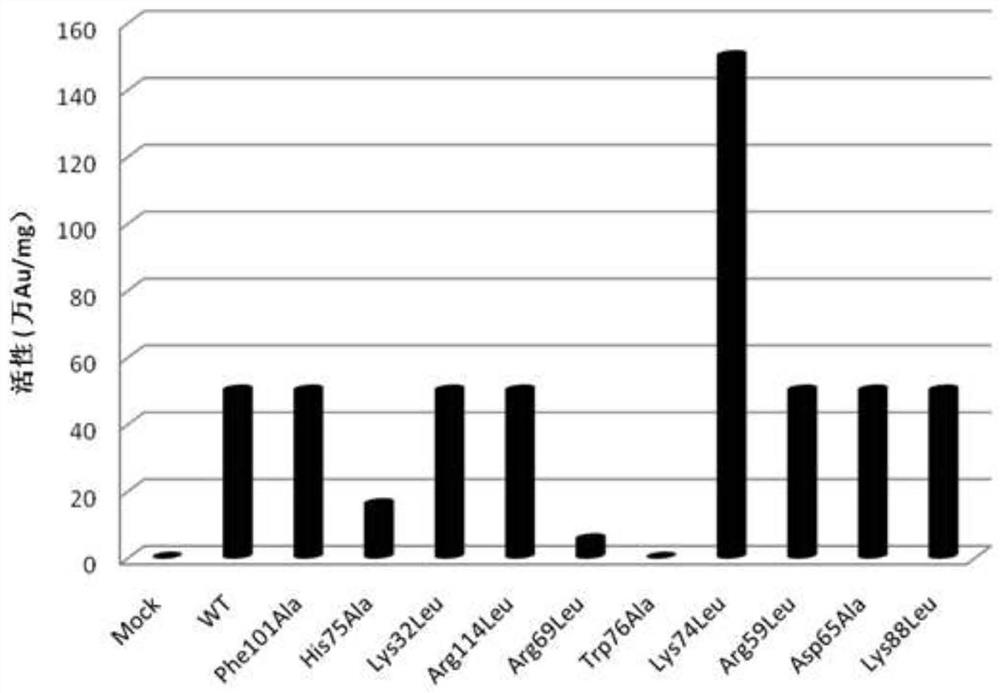
Nerve growth factor mutants
一种神经生长因子、突变体的技术,应用在生物制药领域,能够解决疼痛、NGF严重、使用受限等问题,达到减轻疼痛副作用、生物活性提高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1. Plasmid construction of wild-type hNGF and its mutants
[0063] 1. Construction of the DNA sequence expression plasmid of wild-type hNGF
[0064] Synthesize the DNA sequence of wild-type hNGF (SEQ ID NO: 1 in the sequence listing), and use primers (F: GGAATTCATGTCCATGTTG (SEQ ID NO: 41 in the sequence listing), R: CAAGCTTTCAGGCTCTTCT (SEQ ID NO: 42 in the sequence listing)) to carry out PCR on the target sequence Amplification, after digestion with EcorI (NEB #R0101S), the digestion product was subjected to secondary digestion with HindIII (NEB #R0104S). The pcDNA3.1(-) expression vector was digested using the same method. Carry out agarose gel electrophoresis on the digested vector and the PCR-amplified target fragment, cut out the target fragment, and use a DNA gel recovery kit (TIANGEN, #DP209-03) to recover the digested vector and target DNA fragment respectively. DNA ligase kit (Takara / 6022) was ligated at 16°C for 1 h to complete the plasmid construct...
Embodiment 2
[0067] Example 2. Plasmid transformation and extraction of hNGF and its mutants
[0068] 1. Conversion:
[0069] The hNGF and its mutant plasmids constructed in the above Example 1 were subjected to heat shock transformation. transform. 2ul plasmid was added to it, mixed by flicking, and ice-bathed for 30min. Dry bath at 42°C for 90s, do not shake the centrifuge tube during this period, and immediately place it on ice for 2min. Add 500ul of anti-anti-LB / SOC medium, 150rpm / min, and incubate at 37°C for 45min. Pour all the liquid in the centrifuge tube onto the LB plate and spread it evenly. After the plates were air-dried, they were placed upside down in an incubator for 16 h.
[0070] 2. Plasmid lift
[0071] Pick a single colony obtained in the above 2.1 experiment and inoculate it in 500ul LB liquid medium, cultivate at 37°C for 7h, and send the bacterial liquid to the detection sequence at the same time. Shake the bacteria with the correct sequencing in a large amoun...
Embodiment 3
[0072] Example 3. Expression of wild-type hNGF and its mutants
[0073] The wild-type hNGF and its mutant plasmids obtained in Example 2 above were transfected into 293F cells, and the expression supernatant was collected and quantified on the fourth day after transfection.
[0074] Experimental steps:
[0075] 1. One day before transfection, inoculate 293F cells, 0.5×10 6 / ml, a total of 900ml, 300ml / bottle.
[0076] 2. Count on the day of transfection, and the cell density is about 1.0×10 6 / ml, the viability rate is over 99%.
[0077] 3. Transfection: Take 36ml of cell culture medium into a 125ml culture flask; add 360ug plasmid, mix well; add 1080ug PEI, mix well. Let stand at room temperature for 15 minutes; mix with cells, about 12.3ml / vial; 37℃, 8%CO 2 , 120RPM culture.
[0078] 4. The cell supernatant was collected on the fourth day after transfection, and centrifuged at 10,000g for 20 minutes.
[0079] 5. Collect the supernatant and filter with 0.45um to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com