Preparation method of avibactam sodium
A technology of avibactam sodium and compounds, applied in the direction of organic chemistry, can solve the problems of uncompetitive production cost, long validity period, and affecting the overall yield, and achieve shortened preparation cycle, mild process conditions, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
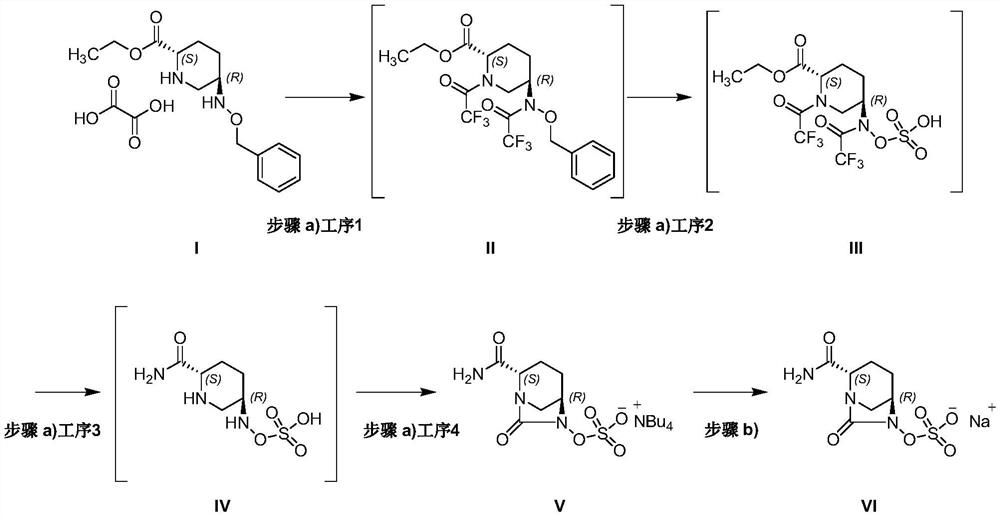
[0042] Example 1 Preparation of compound V
[0043]
[0044] Process 1:
[0045] Compound I (3.68kg, 10.0mol, 1.0eq.) was added to 30L of dichloromethane, sodium carbonate aqueous solution (sodium carbonate: 2.12kg, 20.0mol, 2.0eq.; water: 20L) was added under stirring, and the salt was dissolved with stirring , the liquids were separated, the aqueous phase was extracted with 10 L of dichloromethane, the combined dichloromethane phases were dried over anhydrous sodium sulfate, and filtered. The filtrate was added to the reaction kettle, and triethylamine (2.53kg, 25.0mol, 2.5eq.) was added, and under nitrogen protection, the temperature was lowered to 0-10°C, and trifluoroacetic anhydride (4.62kg, 22.0mol, 2.2eq.) was added dropwise. , after the reaction for 5 hours, the temperature was raised to 20-30 °C for 1 hour, and the reaction was completed by TLC monitoring. The salt was removed by filtration, and the dichloromethane phase was concentrated to obtain the intermedia...
Embodiment 2
[0055] Example 2 Preparation of compound VI
[0056]
[0057] Compound V (3.66kg, 7.2mol, 1.0eq.) was dissolved in a mixed solution of ethanol and water (ethanol: 12.5L; water: 250mL), and ethanol solution of sodium isooctanoate (sodium isooctanoate: 2.36 mL) was added dropwise at 20 to 30°C. kg, 14.4.2mol, 2.0eq.; ethanol: 10L), reacted for 3 hours, filtered to obtain avibactam sodium VI 1.95kg (theoretical value was 2.07kg) as white crystals, and the yield was 94%.
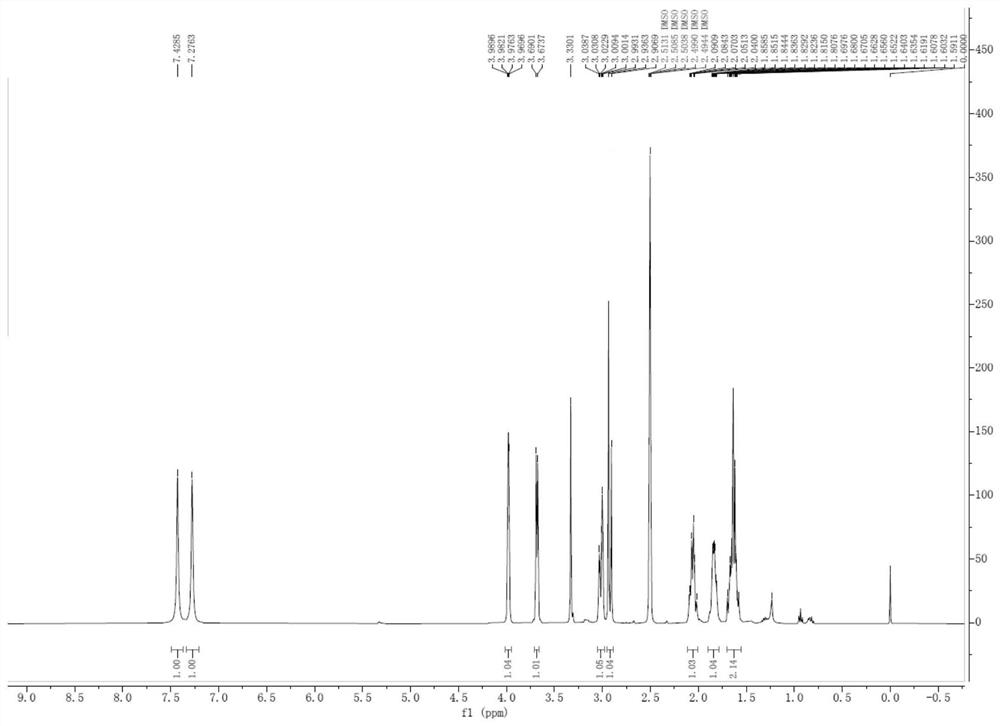
[0058] 1 H NMR (DMSO-d 6 ,400MHz)δ7.42(s,1H),7.27(s,1H),3.96-3.98(m,1H),3.67-3.69(d,1H, J=6.6Hz), 3.03-3.08(m,1H) ,2.90-3.00(m,1H),2.04-2.09(m,1H),1.75-1.83(m,1H),1.59-1.65(m,2H);
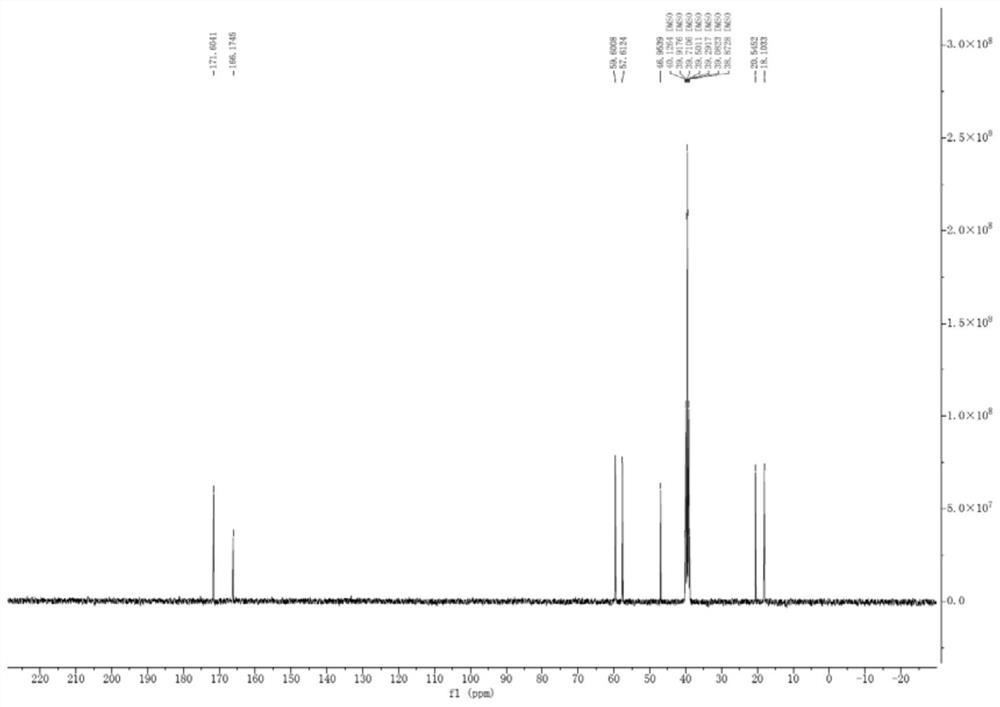
[0059] 13 C NMR (DMSO-d 6 ,100MHz)δ171.6,166.1,59.6,57.6,46.9,20.5,18.1;
[0060] ESI-MS[M] - m / z 264.
Embodiment 3
[0061] Example 3 Preparation of Compound V
[0062] Process 1:
[0063] Compound I (368.4g, 1.0mol, 1.0eq.) was added to 3L of dichloromethane, potassium carbonate aqueous solution (potassium carbonate: 276.4g, 2.0mol, 2.0eq.; water: 2L) was added with stirring, and the salt was dissolved with stirring , the liquids were separated, the aqueous phase was extracted with 1 L of dichloromethane, the combined dichloromethane phases were dried over anhydrous sodium sulfate, and filtered. The filtrate was added to the reaction kettle, triethylamine (253.9g, 2.5mol, 2.5eq.) was added, and under nitrogen protection, the temperature was lowered to 0-10°C, and trifluoroacetic anhydride (462.1g, 2.2mol, 2.2eq.) was added dropwise. , after the reaction for 5 hours, the temperature was raised to 20-30 °C for 1 hour, and the reaction was completed by TLC monitoring. The salt was removed by filtration, and the dichloromethane phase was concentrated to obtain the intermediate compound II as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com