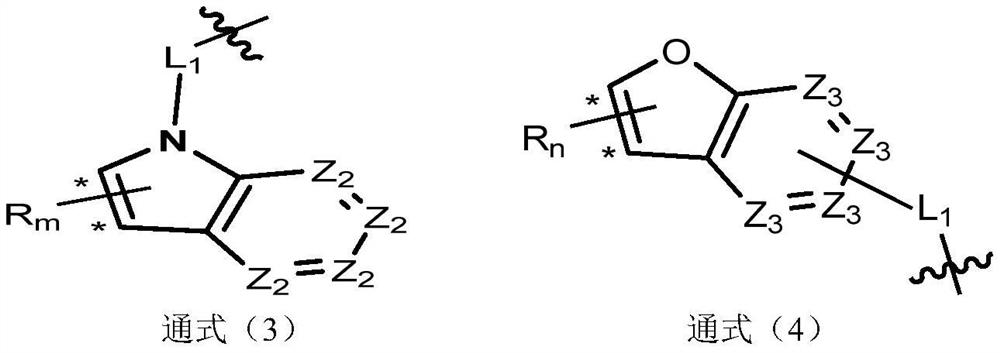Compound taking triazine derivative as core and application thereof
A technology of triazine derivatives and compounds, which is applied in the field of compounds with triazine derivatives as the core, can solve the problems of hole and electron imbalance, shortened lifespan, device efficiency roll-off, etc., achieve high Tg and reduce crystallinity , the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
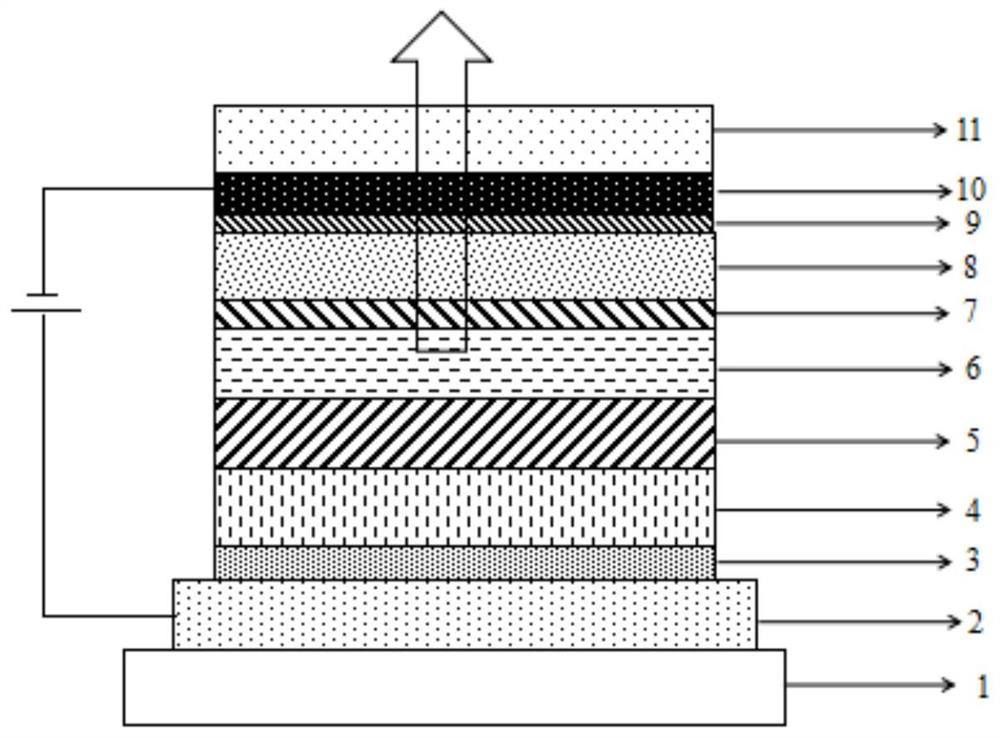
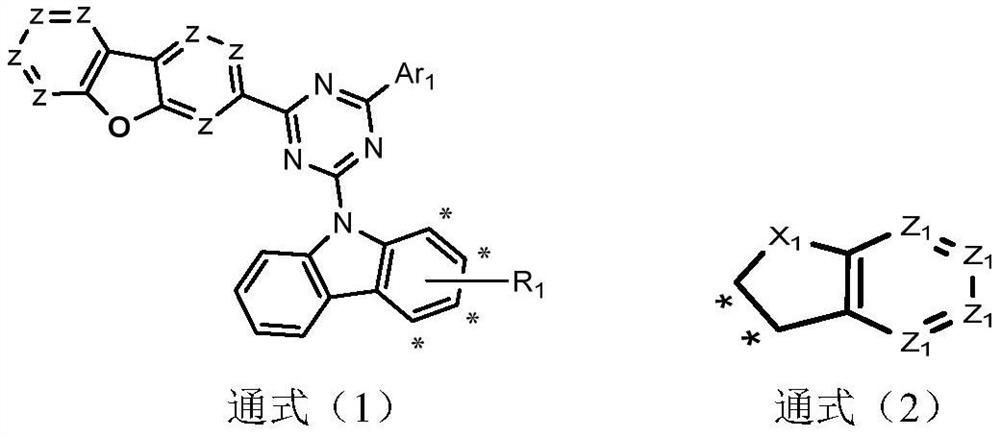
Image
Examples
Embodiment 1
[0081] Example 1: Preparation of Compound 4:
[0082]
[0083] (1) In a three-necked flask, under the protection of nitrogen, add 0.01mol of raw material A1, 0.012mol of raw material B1, and 150ml of toluene, stir and mix, and then add 5×10 -5 mol Pd 2 (dba) 3 , 5×10 -5 mol P(t-Bu) 3 , 0.03mol sodium tert-butoxide, heated to 105 ° C, refluxed for 24 hours, sampling point plate to confirm the reaction is complete; naturally cooled to room temperature, filtered, the filtrate was rotary evaporated to no fraction, passed through a neutral silica gel column to obtain intermediate a1 . LC-MS: found: 612.43 ([M+H] + ), exact mass: 611.15.
[0084] (2) In a three-necked flask, under the protection of nitrogen, add 0.01mol of intermediate a1, 0.012mol of raw material C1, 150ml of toluene, stir and mix, and then add 5×10 -5 mol Pd 2 (dba) 3 , 5×10 -5 mol P(t-Bu) 3 , 0.03mol of sodium tert-butoxide, heated to 105°C, refluxed for 15 hours, and sampled to confirm that the rea...
Embodiment 2
[0085] Example 2: Preparation of Compound 35:
[0086]
[0087] (1) In a three-necked flask, under the protection of nitrogen, add 0.01mol of raw material A1, 0.012mol of raw material B2, and 150ml of toluene, stir and mix, and then add 5×10 -5 mol Pd 2 (dba) 3 , 5×10 -5 mol P(t-Bu) 3 , 0.03mol sodium tert-butoxide, heated to 105 ℃, refluxed for 20 hours, sampling point plate to confirm the reaction is complete; naturally cooled to room temperature, filtered, the filtrate was rotary evaporated to no fraction, passed through a neutral silica gel column to obtain intermediate a2 ; LC-MS: found: 537.22 ([M+H] + ), exact mass: 536.10.
[0088] (2) In a three-necked flask, under the protection of nitrogen, add 0.01mol of intermediate a2, 0.012mol of raw material C2, dissolve with mixed solvent (90ml toluene, 45ml ethanol), then add 1×10 -4 mol Pd (PPh 3 ) 4 , 3mol / L K 2 CO 3 The aqueous solution was heated to reflux for 15 hours and reacted for 15 hours. The sampling p...
Embodiment 6
[0089] Example 6: Preparation of Compound 65:
[0090] Synthesis of Intermediate b-1:
[0091]
[0092]
[0093] (1) Add 0.01mol raw material M1, 0.011mol raw material M2, 0.02mol potassium carbonate, 5×10- 5 mol Pd (PPh 3 ) 4 , then add 250 ml of toluene and 50 ml of ethanol to dissolve it, stir and reflux at 60° C. for 8 hours, and observe the reaction by TLC until the reaction is complete. Cool to room temperature naturally, filter, and spin the filtrate until there is no fraction. The resulting material was purified by silica gel column (hexane:DCM=4:1 as eluent) to give Intermediate I-1. LC-MS: found: 267.12 ([M+H] + ), exact mass: 266.05.
[0094] (2) 0.14 mol of Intermediate I-1, 0.145 mol of pyridine hydrochloride were added to the round-bottomed flask, followed by reflux and stirring at 200° C. for 24 hours. When the reaction was completed, the resultant was cooled to room temperature, and then slowly poured into distilled water, followed by stirring for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com