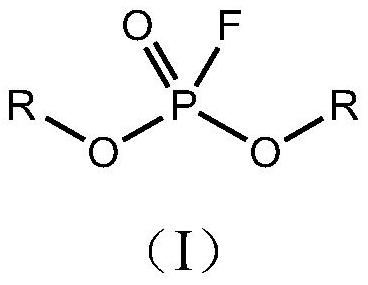
Preparation method of monofluorophosphate
A technology of monofluorophosphate and fluoroalkenyl, which is applied in the field of preparation of high-purity monofluorophosphate, can solve the problems of unfavorable lithium-ion batteries, low purity of additives, and reduced battery capacity and cycle performance, so as to avoid The effect of reducing product yield and improving product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 63.9g (1.1mol) potassium fluoride and 172.5g (1mol) diethyl chlorophosphate to the 500mL there-necked flask at room temperature, under nitrogen protection, heat up to 50°C, stir and react for 4 hours, GC detects the raw material chlorination The content of diethyl phosphate is less than 0.2%, and the content of monofluorodiethyl phosphate in the product is 94.2%.
[0046] KCl and excess potassium fluoride were removed by filtration, the filter cake was washed with 30 g of dichloromethane, the filtrate was combined, 3.1 g of calcium oxide was added to the filtrate, stirred for 60 minutes, and the dichloromethane was recovered by atmospheric distillation, and the distillation was stopped after recovery. The rectification device was changed, and 142.5 g of fractions at 87°C were collected under vacuum of 150 hPa.
[0047] After calculation, the product yield was 91.3%, the GC product purity was 99.8%, and no impurities with a content greater than 0.1% were found.
Embodiment 2
[0049]Add 63.9g (1.1mol) potassium fluoride and 172.5g (1mol) diethyl chlorophosphate to the 500mL there-necked flask at room temperature, under nitrogen protection, heat up to 50 DEG C, stir reaction for 4 hours, GC detects raw material chlorination The content of diethyl phosphate is less than 0.2%, and the content of monofluorodiethyl phosphate in the product is 93.8%.
[0050] KCl and excess potassium fluoride were removed by filtration, the filter cake was washed with 30 g of dichloromethane, the filtrate was combined, 4.6 g of sodium carbonate was added to the filtrate, stirred for 60 minutes, and the dichloromethane was recovered by atmospheric distillation, and the distillation was stopped after recovery. The rectification device was changed, and 140.1 g of fractions at 87°C were collected under vacuum of 150 hPa.
[0051] After calculation, the product yield was 89.3%, the GC product purity was 99.5%, and no impurities with a content greater than 0.1% were found.
Embodiment 3
[0053] Add 63.9g (1.1mol) potassium fluoride and 144.5 (1mol) dimethyl chlorophosphate to the 500mL there-necked flask at room temperature, under nitrogen protection, heat up to 50 ℃, stirring reaction 4 hours, GC detects raw material chlorophosphoric acid The content of dimethyl ester is less than 0.2%, and the content of dimethyl monofluorophosphate in the product is 94.6%.
[0054] The potassium chloride and excess potassium fluoride were removed by filtration, the filter cake was washed with 30 g of dichloromethane, the filtrate was combined, 3.1 g of calcium oxide was added, stirred for 60 minutes, the dichloromethane was recovered by atmospheric distillation, the distillation was stopped after recovery, and the rectification was changed. device, and collect 116.1 g of 79°C fraction under vacuum of 150 hPa.
[0055] After calculation, the product yield was 90.7%, the GC product purity was 99.6%, and no impurities with a content greater than 0.1% were found.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com