Recombinant protein carrying target protein and autonomously entering eukaryotic cell, recombinant expression vector and recombinant bacterium and application
A recombinant protein and target protein technology, applied in the field of fusion proteins, can solve the problems of easy oxidation and discoloration of phospholipids, poor stability of liposomes, and easy precipitation, and achieve the effect of repairing CPD damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
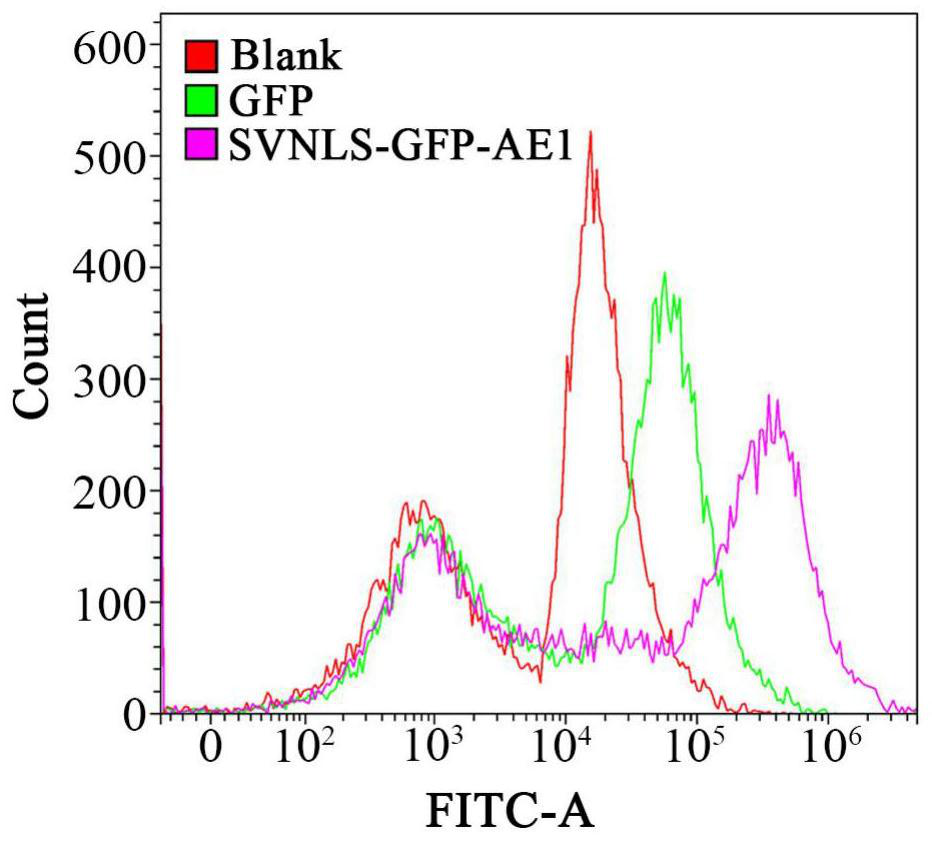
[0058] Analysis of the ability of SVNLS and AE1 to bring the exogenous protein GFP into the nucleus
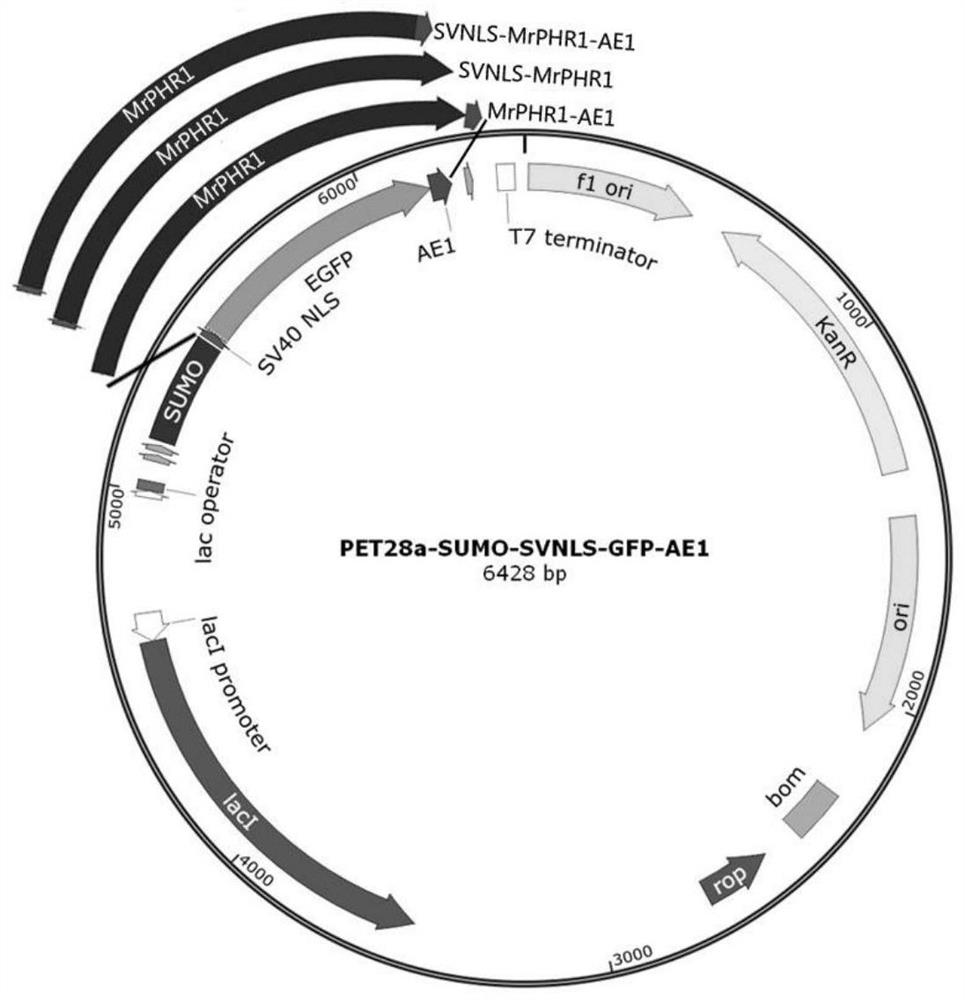
[0059] 1) Construction and prokaryotic expression of SVNLS::GFP::AE1 protein expression vector.
[0060] When the green fluorescent protein GFP coding sequence was cloned by PCR, the coding sequences of SVNLS and AE1 were connected to the N-terminus and C-terminus of the GFP protein, respectively (where the stop codon in the GFP coding sequence was removed and added in front of SVNLS a translation initiation codon ATG).
[0061] Upstream primer SVNLS-GFP-5 (SEQ ID NO. 5) used for PCR amplification: CGGGATCCATGCCAAAAAAGAAGAGAAAGGTCGTGAGCAAGGGCGA GGA; downstream primer AE1-GFP-3 (SEQ ID NO. 6): CGGAATTCTTATCATTTTTTCCATTTCATGCGGCGGTTCTGAAACCAAATTTTAATCTGGCGGCCCTTGTACAGCTCGTCCA. Using the DNA fragment containing the GFP coding sequence as the template, the PCR amplification system (total volume 50 μL) is: ddH 2 O 15 μL, 2×Phanta Maxbuffer (Vazyme) 25 μL, dNTP Mix (10 μM each, Va...
Embodiment 2
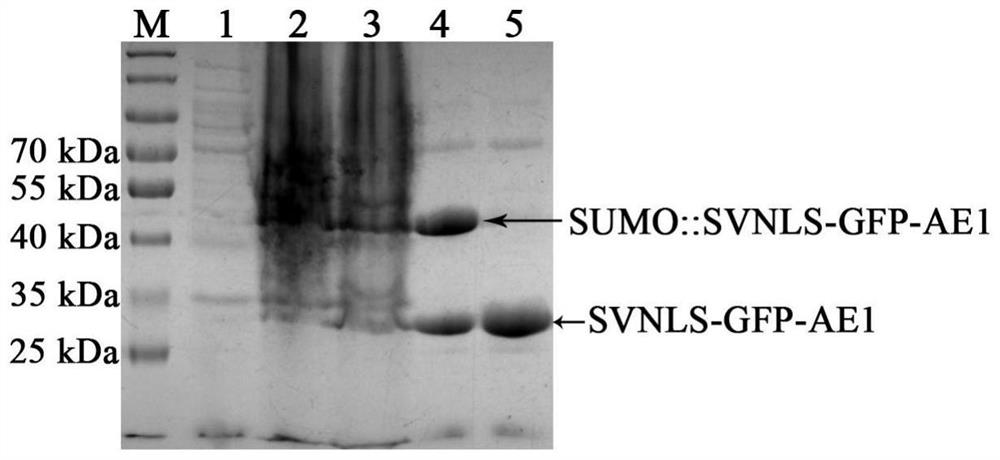
[0074] Expression and preparation of recombinant protein SVNLS::MrPHR1::AE1
[0075] In order to use SVNLS and AE1 short peptide to bring the CPD photolyase (MAA_05216, named MrPHR1) of Metarhizium anisopliae into the nucleus of mammalian cells and repair the CPD damage on nuclear DNA, the present invention constructed a fusion protein SVNLS::MrPHR1:: AE1.
[0076] For this purpose, the coding sequences of SVNLS and AE1 were ligated to the N-terminus and C-terminus of the MrPHR1 protein, respectively, when the MrPHR1 coding sequence (Genbank accession number: XP_007821405) was cloned by PCR (where the stop codon in the MrPHR1 coding sequence was removed). , and add a translation initiation codon ATG in front of SVNLS). The primers used are SEQ ID NO.3 and SEQ ID NO.4, and the template is the cDNA of Metarhizium anisopliae mycelium. The amplification system and amplification procedure are the same as those in Example 1, wherein the upper and lower primers in the amplification...
Embodiment 3
[0084] Analysis of the ability of SVNLS::MrPHR1::AE1 to repair DNA CPD damage after entering the nucleus
[0085] 1) Photorepair of UV-treated HFF-1 cells
[0086] (1) Cell culture and processing setup
[0087] HFF-1 cells were passaged into Corning petri dishes with a diameter of 30 mm, cultured in a carbon dioxide incubator at 37°C for 3 days, and then treated as follows: a negative control without any treatment (untreated), cells were incubated with recombinant protein (Cell+ protein), UV irradiated cells (UV / cell), visible light irradiated UV-treated cells (Light+UV / cell), cells incubated with recombinant protein treated with UV radiation (UV+protein / cell), co-incubated with recombinant protein The incubated cells were UV-irradiated and then treated with visible light (Light+UV+protein / cell), each set in 3 replicates.
[0088] For cells co-incubated with recombinant proteins, UV irradiation followed by visible light treatment (Light+UV+protein / cell) In this setup, there ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com