Isocyanate-based sealing agents and isocyanates protected by said sealing agents
An isocyanate group, blocking isocyanate technology, applied in the field of isocyanate, can solve the problems such as lowering, unrecorded deblocking temperature, and reaction rate constant only slightly increased
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0074] Synthesis Examples, Examples, and Comparative Examples are shown below to describe the present invention in more detail, but the present invention is not limited to the following Examples.
Synthetic example 1
[0076] Synthesis of 3-allyldimethylsilyl propanol
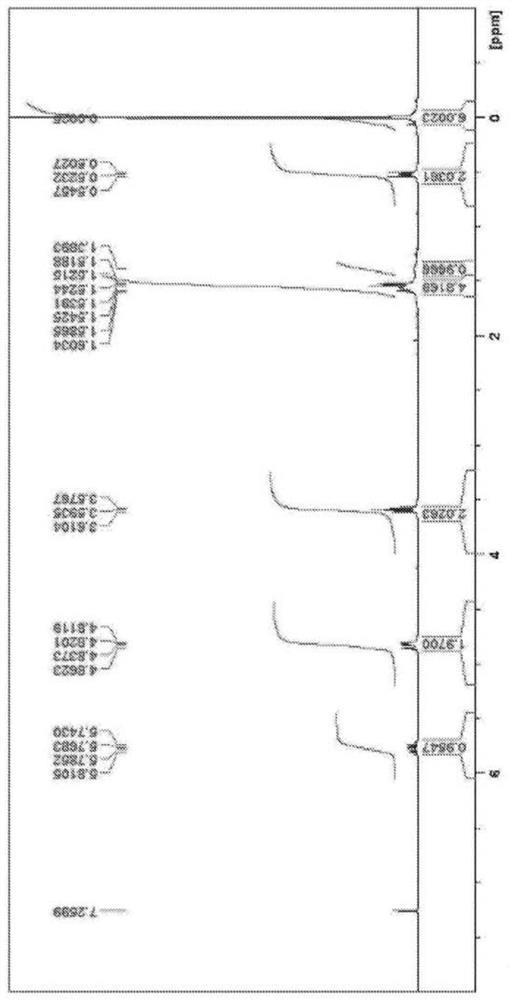
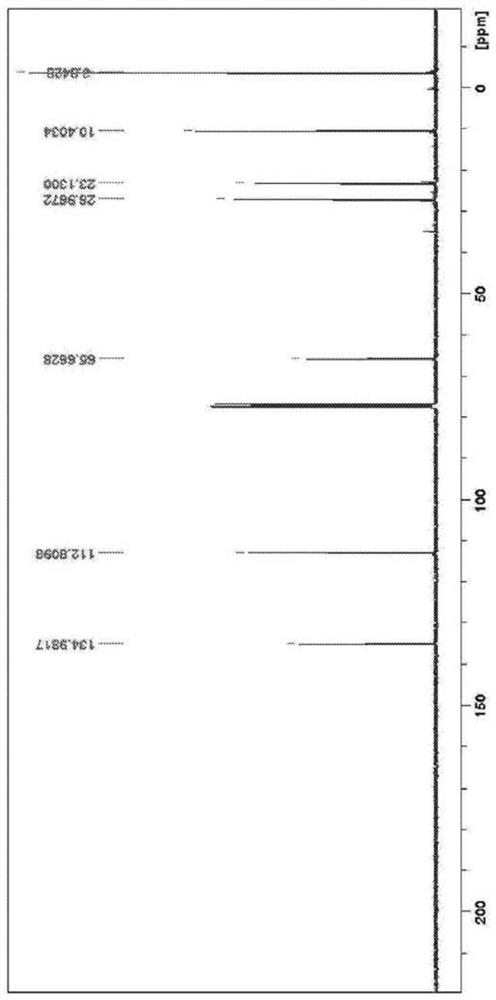
[0077] A 500 ml four-necked flask including a dropping funnel, a Dimroth type cooling condenser, a stirrer, and a thermometer was fully nitrogen-substituted. Diallyldimethylsilane (7.6 g), tetrahydrofuran (THF) (150 ml) were charged and nitrogen purged while cooling the contents of the flask with an ice bath. Next, a 0.5M solution of 9-borabicyclo[3.3.1]nonane (54 ml) was added dropwise. The mixture takes on a clear and uniform appearance. After stirring at room temperature for 3 hours, the contents of the flask were again cooled in an ice bath, and 3M-NaOH aqueous solution (27 ml) and 30% hydrogen peroxide water (33 ml) were added and shaken. After stirring at room temperature for 1 hour, 100 ml of water and 150 ml of hexane were added. The organic layer containing hexane was separated, anhydrous sodium sulfate (20 g) was added, and after filtration, volatile components were removed by a vacuum pump to obtain a transpar...
Synthetic example 2
[0080] Synthesis of 3-(4-methylphenyl)dimethylsilylpropanol
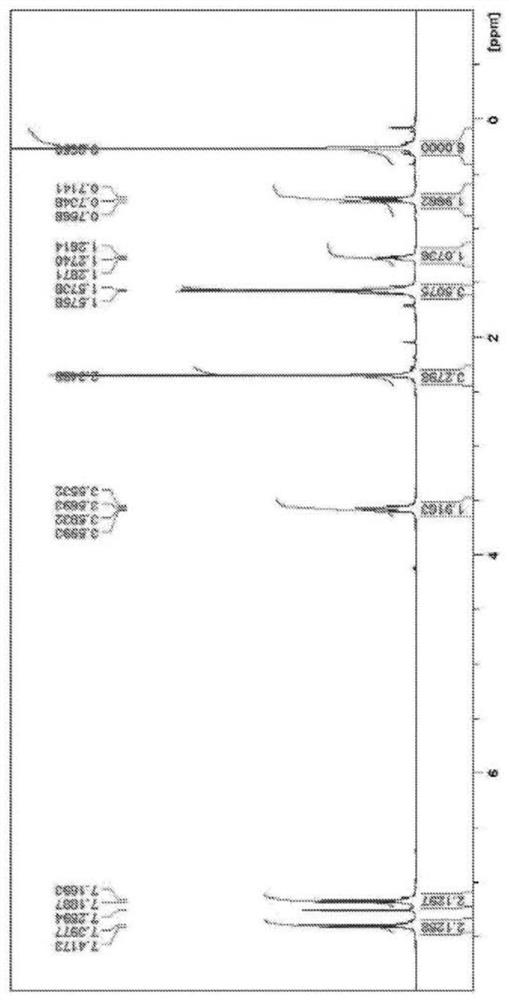
[0081] A 200 ml four-necked flask including a dropping funnel, a Dimroth-type cooling condenser, a stirrer, and a thermometer was fully replaced with nitrogen. Charge allyloxytrimethylsilane (13.0g), 1,5-cyclooctadiene (8ml), di-μ-chlorobis(μ-1,5-cyclooctadiene)diiridium (0.06g) ), and nitrogen ventilation was carried out, while the temperature was raised to 80 °C. Next, chlorodimethylsilane (9.5 g) was added dropwise, and it was made to react at 80 degreeC for 6 hours. A 0.7M THF solution of 4-methylphenylmagnesium bromide was added dropwise thereto, followed by heating under reflux for two hours again. After cooling to room temperature, 100 ml of 1N aqueous hydrochloric acid and 150 ml of ethyl acetate were added. The organic layer containing ethyl acetate was separated, anhydrous sodium sulfate (20 g) was added, and after filtration, the volatile matter was evaporated under reduced pressure. To this, 20 ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com