Purification method of tetanus immune globulin and affinity chromatography filler of tetanus immune globulin
A technology of immunoglobulin and human immunoglobulin, applied in the field of human immunoglobulin purification, can solve the problem of lack of tetanus antibody enrichment, etc., achieve low possibility of clinical side effects, suitable for clinical use, and comprehensive utilization of plasma Enhanced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
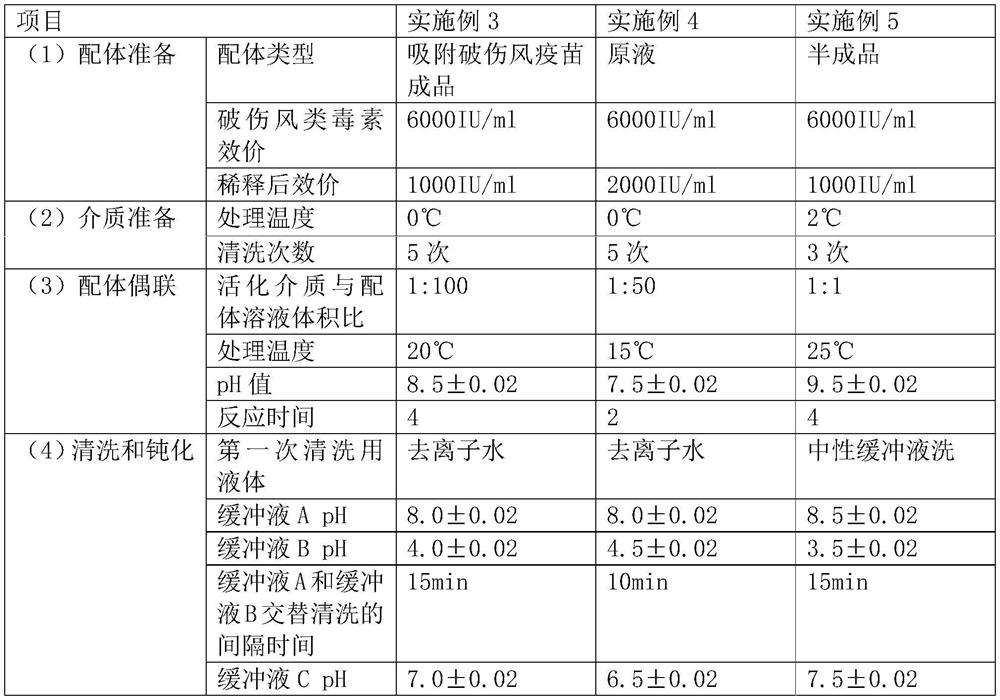
[0033] Example 1: Preparation of chromatography packing
[0034] (1) Ligand preparation: The raw materials of the ligand include finished tetanus toxoid products, semi-finished products and stock solutions, which can be obtained through commercial channels. Among them, tetanus toxoid is the main functional component of tetanus vaccine. Use a 0.2 μm filter element to filter and concentrate the above raw materials, so that the tetanus toxoid titer reaches more than 5000 IU / ml. Dilute the titer to 1000-2000IU / ml with coupling buffer (0.2mol / L sodium bicarbonate+0.5mol / L sodium chloride, pH7.50-8.50) to obtain the ligand solution.
[0035] (2) Medium preparation: The cross-linked agarose-based medium is washed 3-5 times with a 1 mmol / L hydrochloric acid solution at 0-2° C. to obtain an activation medium.
[0036] (3) Ligand coupling: The washed activation medium is mixed with the ligand solution at a ratio of 1:1-1:100 (that is, 1ml of the activated medium is mixed with 1ml-100ml ...
Embodiment 2
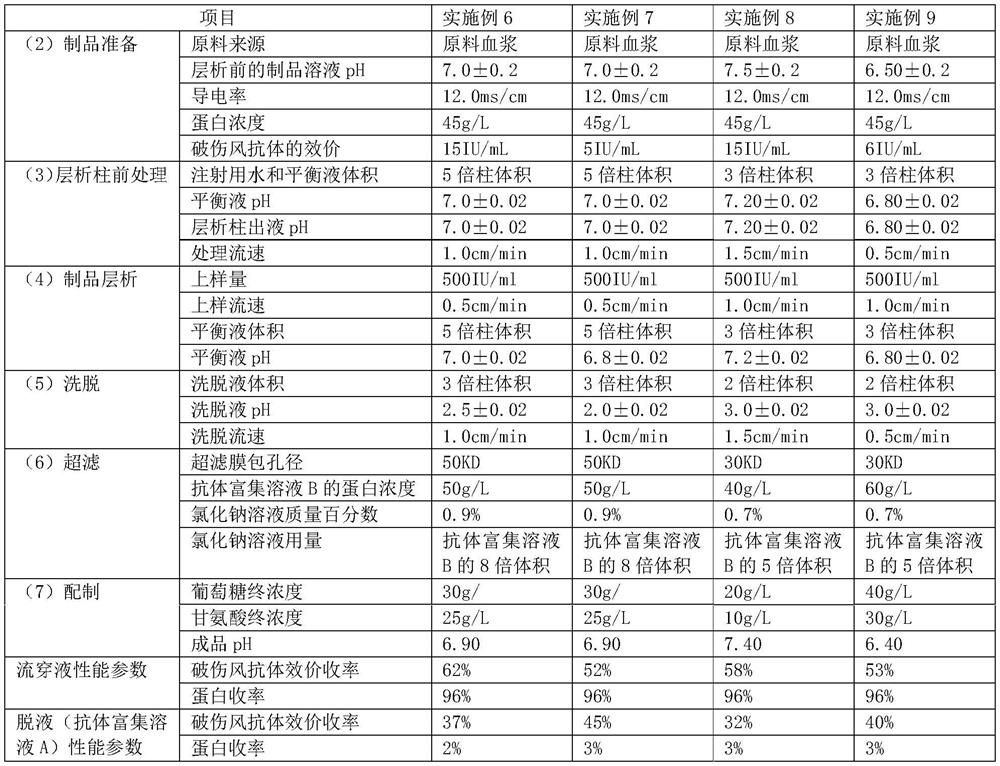
[0038] Example 2: Purification of Tetanus Immunoglobulin
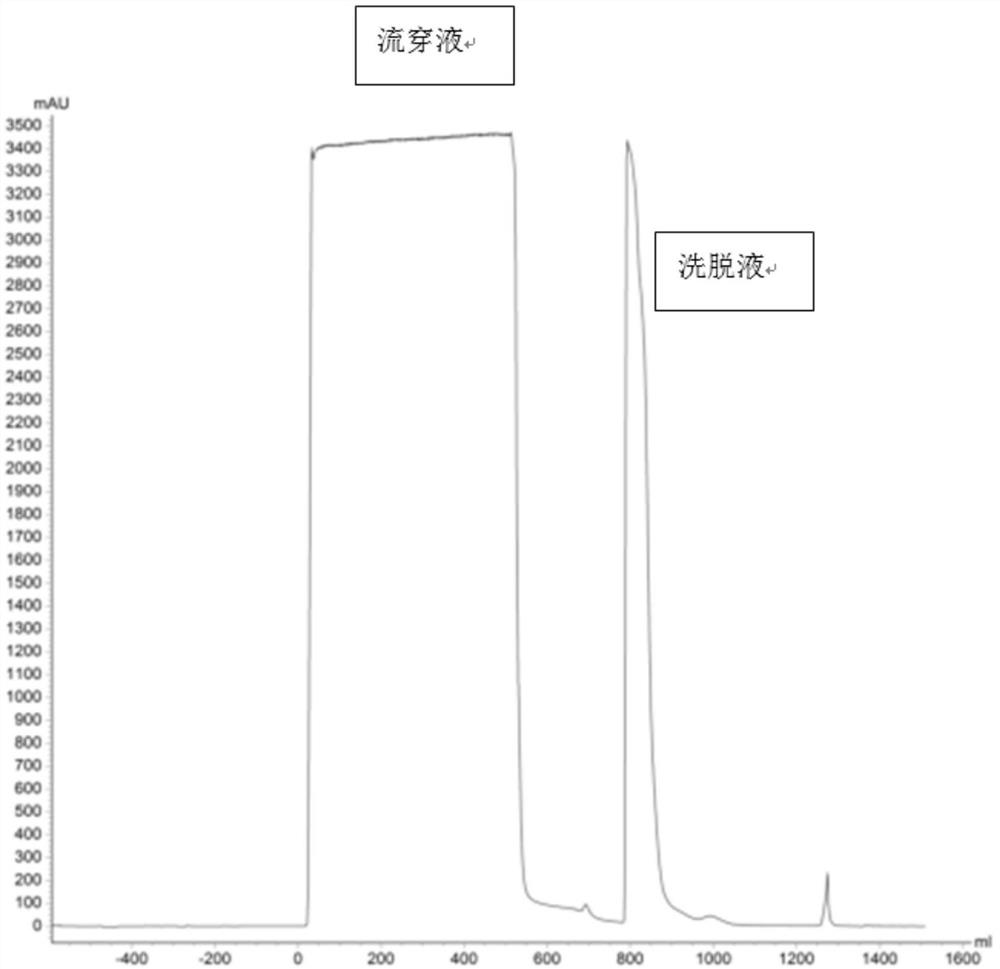
[0039] (1) Chromatography packing (layered medium) packing: Load the equilibrated microspheres into the chromatography column.
[0040] (2) Preparation of products: prepare an immunoglobulin solution containing tetanus antibodies, including raw plasma, cold gel-removed plasma, component II+III, component II, secondary precipitation and other precipitation lysates after filtration, component I, Component III, primary supernatant, etc. after ultrafiltration to remove ethanol, that is, components obtained in each step in the plasma production process can be used as raw materials. If it is in the precipitation state, it needs to be dissolved and filtered, and if the solution contains ethanol, it needs to be removed by ultrafiltration. The immunoglobulin solution containing tetanus antibody also includes the specific human immunoglobulin produced, the intravenous human immunoglobulin stock solution, and the semi-finished o...
Embodiment 10
[0054] Embodiment 10: get the precipitation of component II in the low-temperature ethanol method production process, use 6 times of the precipitation weight to dissolve in water for injection, use a deep filter stack and a terminal to filter the product in series with 0.2 μm after dissolving, and then follow the pH and conductivity of Example 6. The product solution before chromatography is required to be prepared according to the protein concentration, and then chromatography, ultrafiltration and preparation are carried out according to the method of Example 6 to obtain a finished product of tetanus human immunoglobulin.
[0055] The specific acquisition process of component II is as follows:
[0056] After the raw plasma is released from the warehouse, use 75vol.% ethanol solution to sterilize the surface of the plasma bag, then break the plasma bag, control the temperature to melt at 0°C, combine the plasma after melting, centrifuge the plasma using a centrifuge (centrifuga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com