Human extracellular matrix metalloproteinase inducible factor magnetic particle chemiluminescence immunoassay quantitative detection kit and detection method thereof
A technology of chemiluminescence immunity and metalloprotease, which is applied in the direction of chemiluminescence/bioluminescence, analysis by making materials undergo chemical reactions, and measurement devices, can solve problems such as side effects, achieve easy dispersion, solve low sensitivity, and wide detection range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1. Preparation of the kit
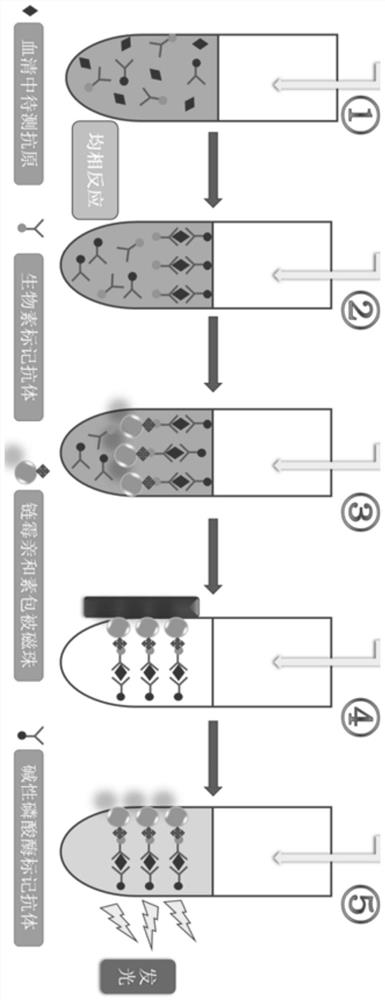
[0033]The magnetic particles of the present invention are used as the solid phase of the immune reaction, and the chemiluminescence immunoassay method is used in conjunction with a chemiluminescence analyzer to measure the content of human extracellular matrix metalloproteinase-inducing factors in human body samples. The kit includes streptavidin-coated magnetic particle suspension, alkaline phosphatase-labeled human extracellular matrix metalloproteinase-inducing factor (CD147) monoclonal antibody (referred to as enzyme-labeled monoclonal antibody), and biotin-labeled human cells Extracellular matrix metalloproteinase inducible factor (CD147) monoclonal antibody (referred to as biotin-labeled monoclonal antibody), human extracellular matrix metalloproteinase inducible factor (CD147) calibrator, etc. The kit according to the invention uses magnetic particles as the solid phase of the immune reaction, and uses the chemiluminescence imm...
Embodiment 2
[0059] Embodiment 2, the test method of test kit
[0060] (1) Sample addition and incubation process: Pipette 50uL of human extracellular matrix metalloproteinase-inducing factor (CD147) calibrator or fresh patient sample into the reaction tube, and then add alkaline phosphatase-labeled human extracellular matrix metalloproteinase-inducing factor ( CD147) monoclonal antibody 50uL and biotin-labeled human extracellular matrix metalloproteinase-inducing factor (CD147) monoclonal antibody 50uL, incubate at 37°C for 10 minutes; then add streptavidin-coated magnetic particle suspension 50uL, Incubate the reaction at 37°C for 10 minutes;
[0061] (2) Magnetic separation and cleaning process: put the reaction tube after the incubation reaction is completed on the magnetic separation rack for 1 minute, and remove the supernatant; add 300uL of magnetic bead coating buffer for the first time, and place it on the magnetic separation rack Let stand for 1 minute, remove the supernatant; a...
Embodiment 3
[0063] Example 3. Performance test results of the kit.
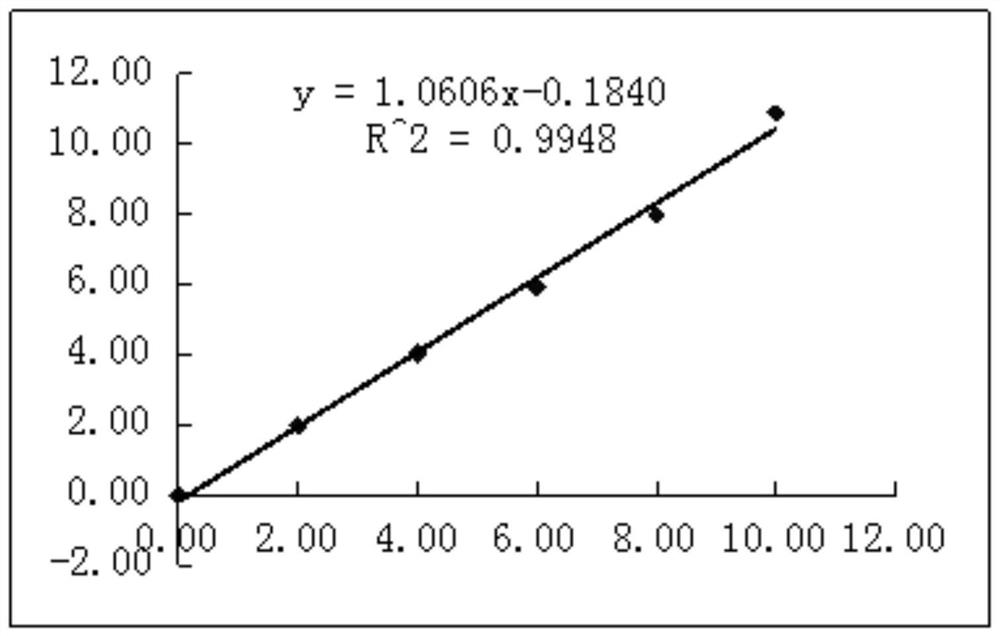
[0064] Kit performance evaluation indicators: The accuracy, linearity, precision and stability of the kit prepared by this method were determined. The standard curve is drawn after detection with protein calibrator. The standard curve is as follows: image 3 As shown, the standard curve formula y=1.0606x-0.1840, and the linearity of the test results is evaluated at the same time. The test results are shown in the following table:
[0065] 1. Accuracy test results
[0066]
[0067] 2. Linearity test results
[0068]
[0069] 3. Precision test results
[0070] repeat times low value samples 1 0.49 2 0.50 3 0.50 4 0.51 5 0.52 6 0.51 7 0.52 8 0.52 9 0.49 10 0.51 average value 0.51 Coefficient of Variation CV 2.38%
[0071] 4. Stability test results
[0072]
[0073] 5. Sensitivity test results
[0074]
[0075] It can be seen from...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com