Method and catalyst for carrying out selective nitro reduction hydrogenation reaction by using micro-packed bed
A hydrogenation reaction and catalyst technology, which is applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of low mass transfer efficiency, high cost, long reaction time, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
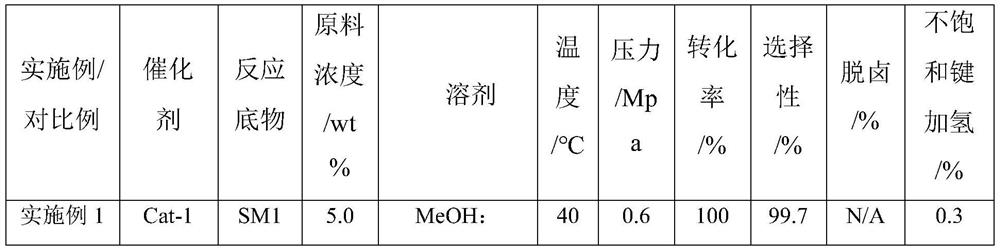
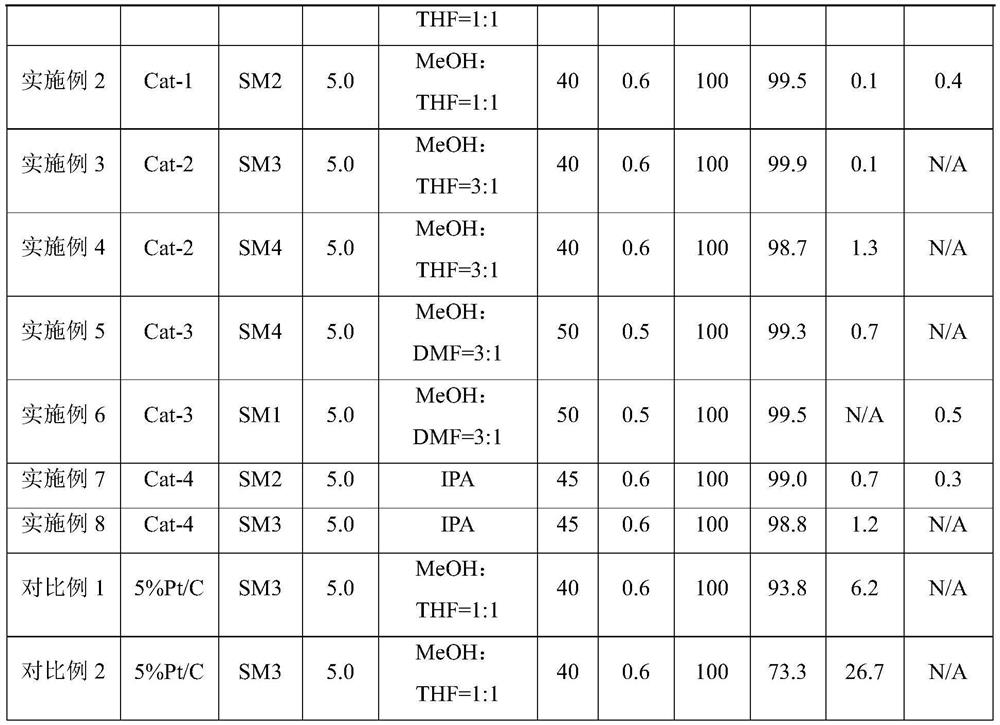
[0034] Preparation of catalyst Cat-1: chloroplatinic acid (2.71 g) and cobalt nitrate (0.95 g) were weighed and dissolved in distilled water (36.5 mL). Weigh the activated carbon carrier (50 g), slowly add the above impregnating solution dropwise onto the carrier, and add dropwise while stirring until all the impregnating solution is dripped. After aging for 12h, put it in an oven at 120°C for 4h. Then, it was put into a muffle furnace for calcination at 450 °C for 4 h to obtain a catalyst precursor. The catalyst precursor was placed in a tube furnace, and hydrogen was introduced to reduce it at 400°C for 10 hours. After the catalyst was lowered to room temperature, the catalyst Cat-1 was prepared.
[0035] The reaction substrate SM1 (10.0 g) was taken and dissolved in a mixed solution of methanol (100 g) and tetrahydrofuran (100 g). Put the beaker of the mixed solution into an ultrasonic apparatus and ultrasonically at room temperature for 30 minutes until it is completely ...
Embodiment 2
[0037] The reaction substrate SM2 (10.0 g) was taken and dissolved in a mixed solution of methanol (100 g) and tetrahydrofuran (100 g). Put the beaker of the mixed solution into an ultrasonic apparatus and ultrasonically at room temperature for 30 minutes until it is completely dissolved, and prepare a raw material solution with a mass concentration of 5%. Measure the catalyst Cat-1 (5.0 mL) with a graduated cylinder, put it into the reaction tube of the micro-packed bed, tap the reaction tube with a mallet to ensure that the catalyst is packed tightly, and then tighten the ferrules at both ends of the reactor. The reaction conditions were as follows: the reaction temperature was 40° C.; the reaction pressure was 0.6 MPa; the hydrogen flow rate was 20 mL / min; the liquid feed flow rate was 0.3 mL / min. After 2 hours of reaction, sampling was performed to measure the components and contents in the reaction product by liquid chromatography. The chromatographic measurement results...
Embodiment 3
[0039] Preparation of catalyst Cat-2: chloroplatinic acid (2.71 g) and iron nitrate (2.23 g) were weighed and dissolved in distilled water (36.5 mL). Weigh the activated carbon carrier (50 g), slowly add the above impregnating solution dropwise onto the carrier, and add dropwise while stirring until all the impregnating solution is dripped. After aging for 12h, put it in an oven at 120°C for 4h. Then, it was put into a muffle furnace for calcination at 450 °C for 4 h to obtain a catalyst precursor. The catalyst precursor was placed in a tube furnace, and hydrogen was introduced to reduce it at 500°C for 10 hours. After the catalyst was lowered to room temperature, the catalyst Cat-2 was prepared.
[0040] The reaction substrate SM3 (10.0 g) was taken and dissolved in a mixed solution of methanol (150 g) and tetrahydrofuran (50 g). Put the beaker of the mixed solution into an ultrasonic apparatus and ultrasonically at room temperature for 30 minutes until it is completely dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com