Novel allyl hydroximic acid derivative containing sulfonate structure as well as preparation method and application of novel allyl hydroximic acid derivative
A technology of propenyl hydroxamic acid and sulfonate ester, which is applied in the direction of sulfonate ester preparation, drug combination, organic chemistry, etc., to achieve the effect of improving biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
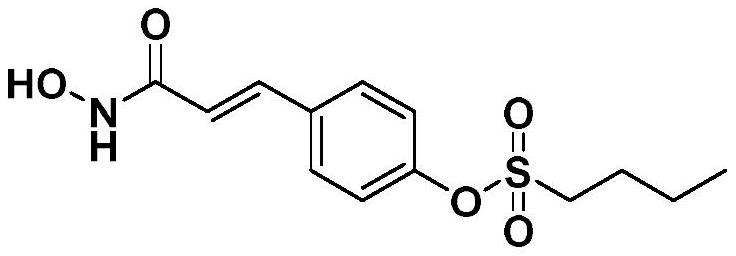
[0029] 4-(3-(Hydroxyamino)-3-oxopropyl-1-en-1-yl)phenylbutane-1-sulfonate
[0030]
[0031] It is called spartamin G1.
[0032] Dissolve p-coumaric acid (1 mmol) in a mixed solution of 10 mL of dichloromethane and 1 mL of N,N-dimethylformamide, add n-butylsulfonyl chloride, stir under ice bath conditions for 30-60 min, add dicyclohexyl Carbodiimide (1.5 mmol) and 4-dimethylaminopyridine (0.5 mmol) were moved to room temperature and stirred for 4-12 h, followed by thin layer chromatography. After the reaction, the precipitate was filtered, and the filtrate was extracted with ethyl acetate , washed with saturated sodium chloride solution, distilled off the organic solvent under reduced pressure, recrystallized with a mixed solvent of N,N-dimethylformamide and ethanol, and the obtained compound was dissolved in a mixed solution of 10 mL of isopropanol and 1 mL of piperidine, Hydroxylamine hydrochloride (1 mmol) was added, stirred for 4-8 h under ice bath conditions, the organ...
Embodiment 2
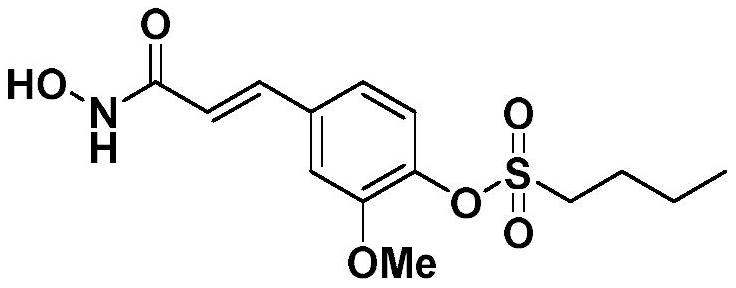
[0035] 4-(3-(Hydroxyamino)-3-oxopropyl-1-en-1-yl)-2-methoxyphenylbutane-1-sulfonate
[0036]
[0037] It is called Spartina G2.
[0038] The preparation method refers to Example 1, the initial raw material of the reaction is ferulic acid, and the sulfonyl chloride is n-butylsulfonyl chloride.
[0039] The final product is a white powder, the yield is 37.1%, the melting point is 109-110°C, 1 H NMR (600MHz, DMSO-d 6 )δ10.79(s,1H,-OH),9.09(s,1H,-NH-),7.47(d,J=15.8Hz,1H,=CH-),7.40(s,1H,-ArH), 7.31(d,J=8.3Hz,1H,-ArH),7.20(d,J=8.2Hz,1H,-ArH),6.51(d,J=15.8Hz,1H,-CO-CH=),3.89( s,3H,-OCH3 ),3.54-3.44(m,2H,-CH 2 -),1.84-1.79(m,2H,-CH 2 -),1.48-1.42(m,2H,-CH 2 -),0.92(t,J=7.4Hz,3H,-CH 3 ). 13 C NMR (151MHz, DMSO-d 6 )δ162.98,152.01,138.76,137.85,135.45,124.72,120.69,120.33,112.88,56.49,51.04,25.70,21.14,13.87.HRMS(ESI-TOF)m / z:[M+H] + Calcd.for C 14 H 20 NO 6 S 330.1011, Found 330.1010.
Embodiment 3
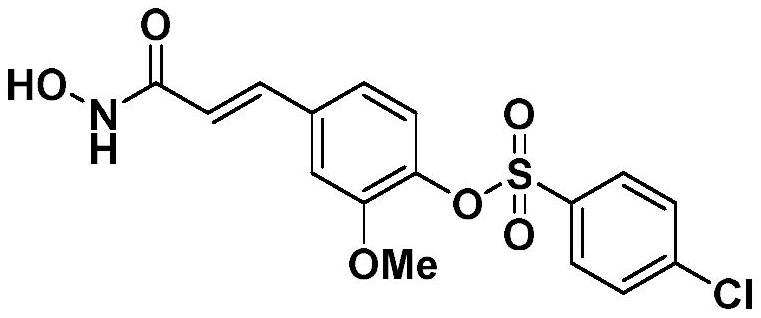
[0041] 4-(3-(Hydroxyamino)-3-oxopropyl-1-en-1-yl)-2-methoxyphenyl-4-chlorobenzenesulfonate
[0042]
[0043] It is called Spartina G3.
[0044] The preparation method refers to Example 1, the initial raw material of the reaction is ferulic acid, and the sulfonyl chloride is 4-chlorobenzenesulfonyl chloride.
[0045] The final product was a white powder with a yield of 35.8% and a melting point of 148-149°C. 1 H NMR (600MHz, DMSO-d 6 )δ10.80(s,1H,-OH),9.10(s,1H,-NH-),7.86-7.84(m,2H,-ArH),7.75(d,J=8.7Hz,2H,-ArH) ,7.44(d,J=15.8Hz,1H,=CH-),7.28(s,1H,-ArH),7.18(s,2H,-ArH),6.49(d,J=15.8Hz,1H,-CO -CH=),3.55(s,3H,-CH 3 ). 13 C NMR (151MHz, DMSO-d 6 )δ162.92,151.76,140.39,138.26,137.68,135.94,134.25,130.62,130.07,124.46,120.99,120.29,112.82,56.05.HRMS(ESI-TOF)m / z:[M+H] + Calcd.for C 16 H 15 ClNO 6 S 384.0308, Found 384.0311.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com