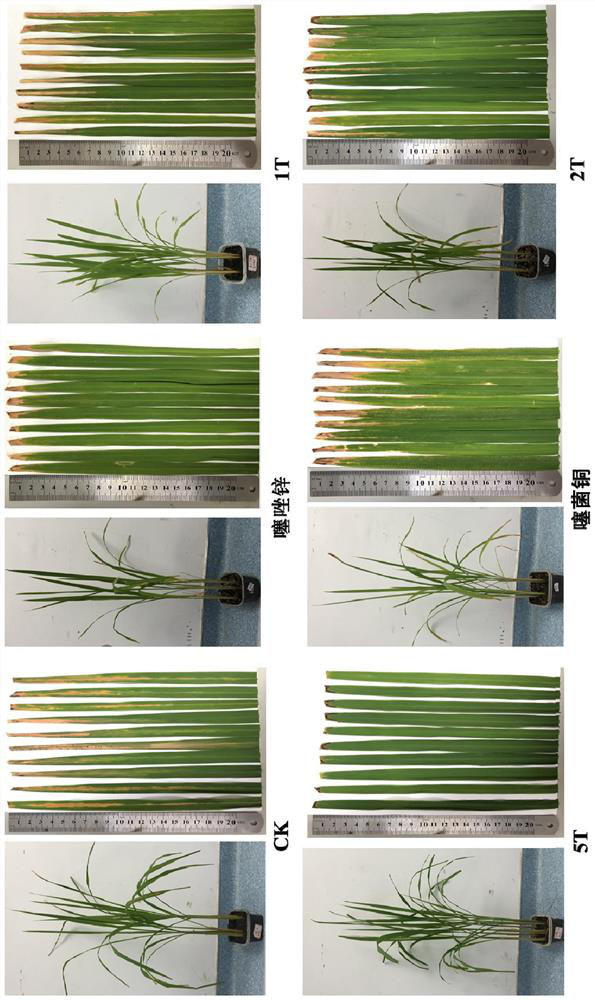
Bis-(1, 3, 4-thiadiazole) derivative as well as preparation method and application thereof
A technology of thiadiazoles and compounds, applied in the field of bis-class derivatives and their preparation, can solve problems such as copper poisoning and red spider induction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The preparation method of the bis-(1,3,4-thiadiazole) compound of the present invention comprises the following steps:
[0060] First, after completely dissolving hypophosphite in water and concentrated hydrochloric acid, put into dithiourea, and directly heat, stir and reflux for 15-20 hours. Washing with water, draining and drying in an infrared oven to obtain 2-amino-5-mercapto-1,3,4-thiadiazole; with the intermediate 2-amino-5-thio-1,3,4-thiadiazole Diazole as raw material, add 2-amino-5-sulfanyl-1,3,4-thiadiazole to the aqueous solution of sodium hydroxide, stir to dissolve all the raw materials, slowly add various dialdehyde solutions, and at room temperature The reaction was carried out for 1-2 hours, then dilute hydrochloric acid was added to adjust the pH value of the system to neutrality, and finally the precipitated solid was vacuum filtered, sucked dry, and dried at 100°C.
[0061] Wherein, the concentration of the sodium hydroxide aqueous solution is 5-20%...
Embodiment 1
[0086] Example 1 Preparation of intermediate 2-amino-5-mercapto-1,3,4-thiadiazole
[0087]In a 500 ml clean three-necked flask, add 50 ml of water and 100 ml of concentrated hydrochloric acid, and 1.5 g of calcium hypophosphite as a catalyst. After stirring and dissolving, slowly add 30 g of dithiourea to the three-necked flask, and heat to 100°C. Carry out reflux reaction, stir and reflux for 15 hours, after the reaction is completed, after the reaction system is cooled to room temperature, crystals are precipitated, and the precipitated crystals are subjected to suction filtration with a glass funnel and then dried under infrared oven conditions to obtain Intermediate 2 -Amino-5-mercapto-1,3,4-thiadiazole 18.35g, the melting point is 230-232°C, the content is 95-97%, and the yield is 87.07%.
Embodiment 2
[0088] Example 2 Preparation of N,N-ethylene-bis(2-amino-5-mercapto-1,3,4-thiadiazole) (1T) compound
[0089] Weigh 5 g (37.54 mmol) of the intermediate 2-amino-5-sulfanyl-1,3,4-thiadiazole into a 250 mL beaker, slowly add 10-20% aqueous sodium hydroxide solution to the beaker, After stirring to dissolve all the raw materials, slowly add 40% glyoxal solution to the beaker, react at 25-30 ° C for 5-6 hours, then add dilute hydrochloric acid to adjust the pH value of the system to neutrality, and finally remove the precipitated The solid was vacuum filtered, sucked dry, and dried at 100°C to obtain 8.73 g of N,N-ethylene-bis-(2-amino-5-mercapto-1,3,4-thiadiazole) compound, The melting point is 129-131°C, the content is 93-95%, and the yield is 79.53%. 1 HNMR (500MHz, DMSO-d 6 )δ13.28(s,-SH,2H),8.22(s,-NH,2H),3.47(t,J=1.36Hz,- (CH 2 ) -,4H). 13 C NMR (100MHz, DMSO-d 6 )δ184(s),164.2(s),48.7(s).HRMS(ESI)m / z for C 6 H 8 N 6 NaS 4 [M+Na] + calcd 314.97431, found: 314.977...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com