Method for measuring concentration of aprepitant in plasma by liquid chromatography-mass spectrometry
A prepitant and liquid mass spectrometry technology, applied in the field of medicine, can solve the problems of not being suitable for large-scale detection of samples, long detection time, and complexity, and achieve the speed of domestic substitution, short detection time, and stable baseline Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Experimental materials and analytical equipment
[0058] Aprepitant (analyte): U.S. Pharmacopeial Convention
[0059] Aprepitant-d4 (internal standard): Toronto Research Chemicals
[0060] The reagents used are shown in Table 1 below:
[0061] Table 1 Reagent details
[0062] Reagent name level manufacturer Acetonitrile (ACN) HPLC J.T. Baker Ammonium acetate (CH3COONH4) HPLC J.T. Baker Methanol (MeOH) HPLC J.T. Baker Ascorbic acid (VC) ACS SIGMA-ALDRICH Hydrochloric acid (HCl) AR Greagent
[0063] Note: Reagents of the same grade or higher can also be used
[0064] The analytical equipment used is shown in Table 2 below:
[0065] Table 2 Details of equipment used
[0066] components type manufacturer Binary pump AD Pump SHIMADZU Degasser (Degasser) Degasser SHIMADZU Column oven AD Column oven SHIMADZU Autosampler AC Autosampler SHIMADZU Sa...
Embodiment 2
[0068] Embodiment 2 Liquid phase conditions
[0069] 1. Liquid chromatography conditions:
[0070] Chromatographic column: Agilent ZORBAX XDB-C18, 5μm, column size is 2.1×50mm; Column temperature: 40℃; Mobile phase A: H 2 O / FA=100 / 0.2(V / V); Mobile phase B: ACN / FA=100 / 0.2(V / V); Wash solution: MeOH / H 2 O=50 / 50 (V / V); the temperature of the autosampler is 15°C; the gradient elution, the flow rate is 0.4000mL / min, the injection volume is 10μL, and the analysis time is 3.00min;
[0071] Table 3 Gradient elution procedure
[0072] Total time (min) Flow rate (ml / min) Mobile phase A (%) Mobile phase B (%) 0.00 0.4000 45.0 55.0 0.60 0.4000 45.0 55.0 0.61 0.4000 20.0 80.0 1.20 0.4000 20.0 80.0 1.21 0.4000 45.0 55.0 3.00 0.4000 45.0 55.0
[0073] 2. Mass spectrometry conditions
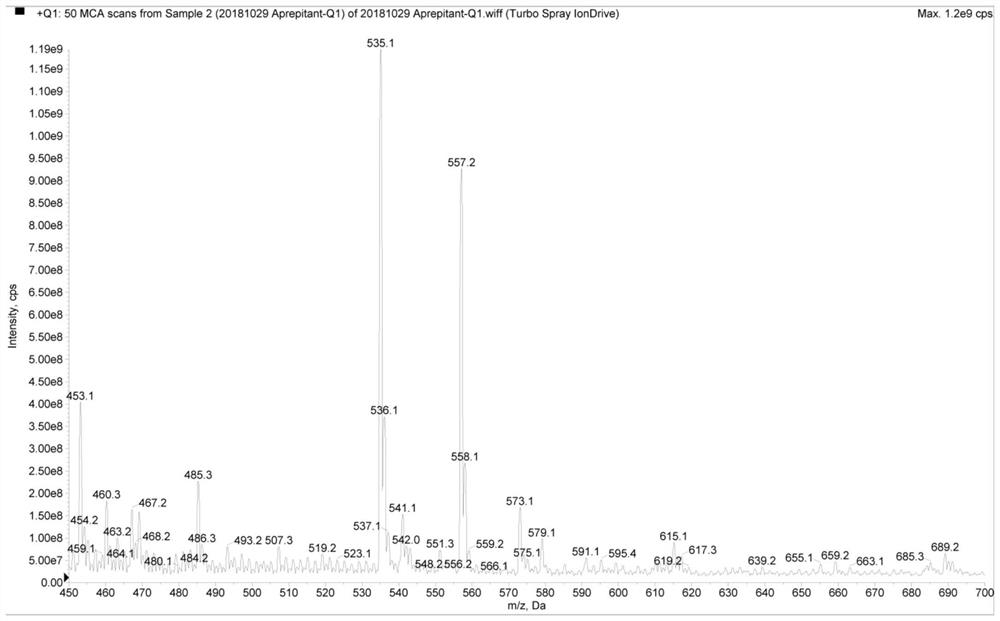
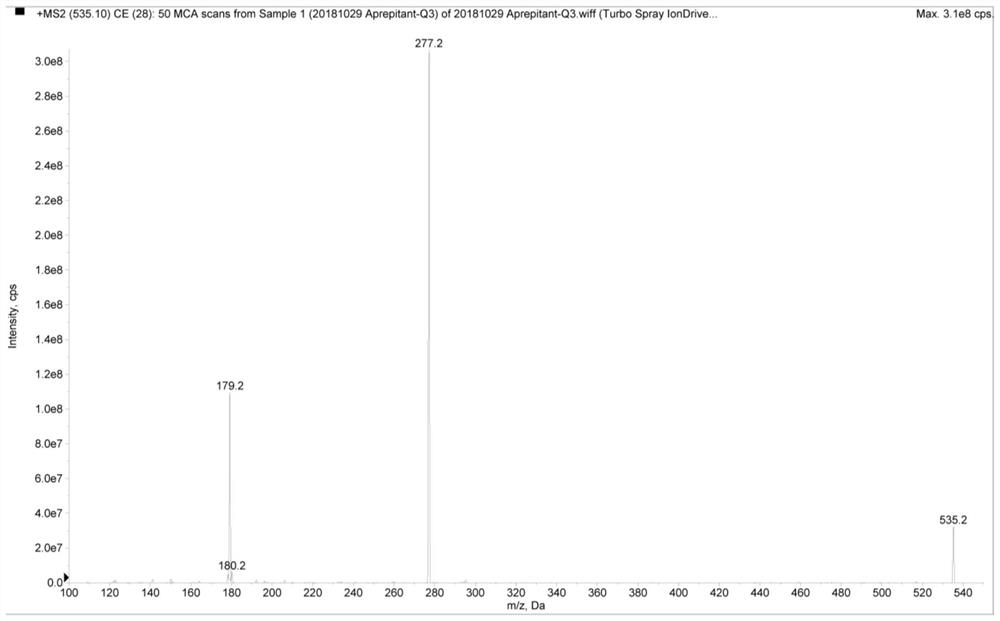
[0074] Aprepitant conditions are:
[0075] The ion source adopts electrospray ion source, the spray voltage is 5500V, the atomization temperatur...
Embodiment 3
[0079] Example 3 Test process
[0080] 1. Preparation of aprepitant standard solution
[0081] Preparation of aprepitant standard solution: Accurately weigh 5.032 mg of aprepitant (analyte), add MeOH to dissolve to 500000ng / mL, and use MeOH / H in turn 2 O=50 / 50 (V / V) solution is diluted to prepare aprepitant standard solution, and the specific dilution concentration is shown in Table 4 below:
[0082] Table 4 Aprepitant standard solution preparation concentration
[0083]
[0084]
[0085] a: Prepared directly from aprepitant
[0086] b: Dilute solution of analyte: MeOH / H 2 O=50 / 50(V / V)
[0087] Aprepitant standard solution is stored in a brown glass container and refrigerator (-20°C) when not in use, and the volume can be increased or decreased proportionally as needed.
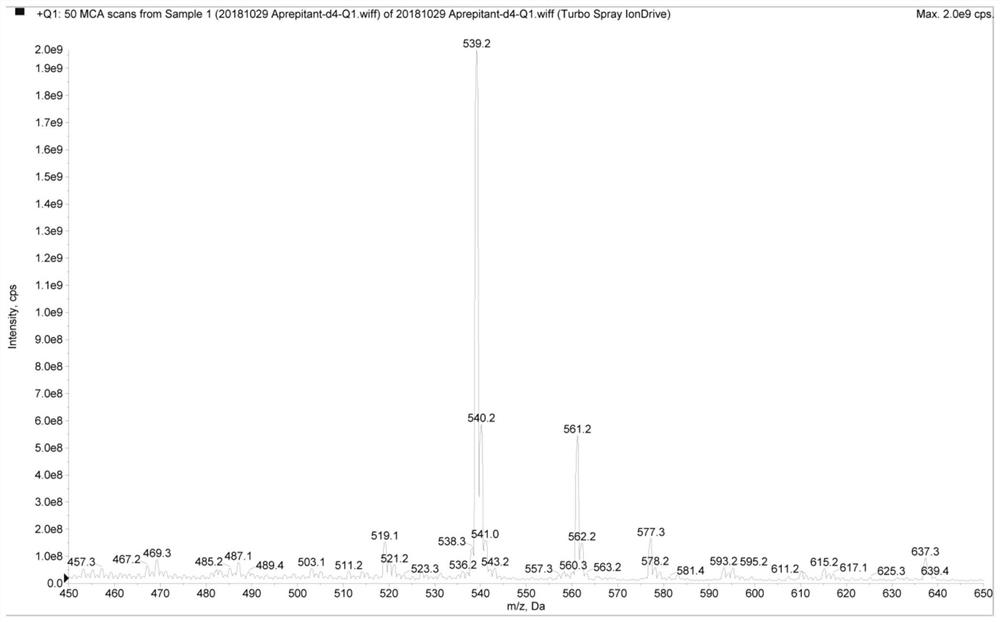
[0088] 2. Preparation of aprepitant-d4 internal standard standard solution
[0089] Preparation of aprepitant-d4 internal standard standard solution: Accurately weigh 1.107 mg of aprepitant-d4 (in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com