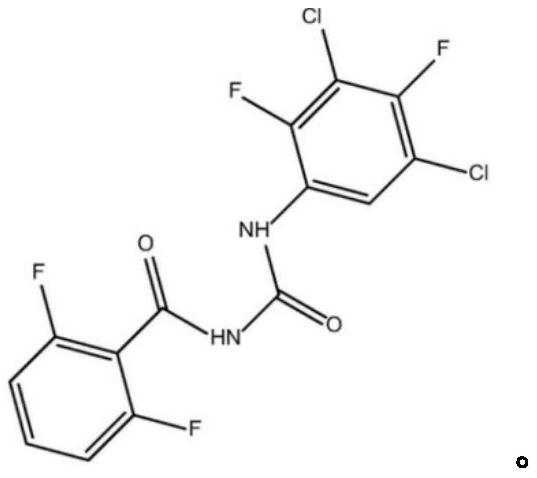
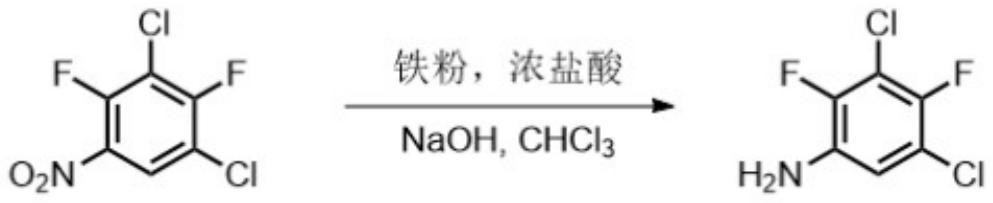
Preparation method of 2, 4-difluoro-3, 5-dichloroaniline
A technology of dichloroaniline and dichlorobenzamide, applied in rearrangement reaction preparation, resistance to vector-borne diseases, organic chemistry, etc., can solve the problems of little industrial significance, undisclosed nitration reaction steps, and impact on intermediates, etc. , to achieve the effects of green reaction process, easy scale-up production, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] A general aspect of the present invention provides a preparation method of 2,4-difluoro-3,5-dichloroaniline, which is characterized in that comprising the following steps:
[0037] Step (1), 2,3,4,5-tetrachlorobenzoyl chloride reacts with ammonia water or ammonia gas to obtain 2,3,4,5-tetrachlorobenzamide;
[0038] step (2), dehydrating the 2,3,4,5-tetrachlorobenzamide prepared in step (1) in the presence of a dehydrating agent to generate 2,3,4,5-tetrachlorobenzonitrile;
[0039] In step (3), 2,3,4,5-tetrachlorobenzonitrile prepared in step (2) is reacted with KF to prepare 2,4-difluoro-3,5-dichlorobenzonitrile;
[0040] Step (4), hydrolyzing the 2,4-difluoro-3,5-dichlorobenzonitrile prepared in step (3) to obtain 2,4-difluoro-3,5-dichlorobenzamide;
[0041] Step (5), 2,4-difluoro-3,5-dichlorobenzamide prepared in step (4) is synthesized into 2,4-difluoro-3,5-dichloroaniline under the action of an oxidant .
[0042] The condition reaction of the route of the inventi...
Embodiment 12
[0062] Implement the synthesis of 12,3,4,5-tetrachlorobenzamide
[0063] Add 0.4L of ammonia water (28% ammonia water solution) to a 4L round-bottomed flask, and then dropwise add a dichloromethane solution of 2,3,4,5-tetrachlorobenzoyl chloride (1.6mol) at a low temperature of -10°C (1L), the temperature was slowly raised to room temperature after the addition, and the reaction was continued at room temperature for 2h. Then the reactant was filtered to obtain a solid, and the solid was washed twice with water to obtain a white solid product, 2,3,4,5-tetrachlorobenzamide, which was dried in vacuo to obtain a product with a yield of 96% and a HPLC purity of 98.5%.
[0064] Example 2-5 adopts the reaction conditions similar to Example 1 to prepare 2,3,4,5-tetrachlorobenzamide, only changing the reaction time, the results are shown in Table 1:
[0065] Table 1 Results of amidation reactions with different reaction times
[0066] serial number Reaction time Yield ...
Embodiment 62
[0067] Example 62, Synthesis of 3,4,5-tetrachlorobenzonitrile
[0068] Add amide (0.8mol, 207.2g) and POCl to a 1L round-bottom flask 3 (400 mL), the reaction was carried out at 110 °C for 3 h. Evaporate excess POCl under reduced pressure 3 , cooled to room temperature, and ice water was added to quench the residual POCl 3 , filtered, and washed the filtrate twice with water (100ml of water each time) to obtain a white solid product, which was dried in vacuo to obtain the product 2,3,4,5-tetrachlorobenzonitrile, the yield was 86.8%, and the HPLC purity was 96.3%.
[0069] In Examples 7-10, 2,3,4,5-tetrachlorobenzonitrile was prepared under substantially the same conditions as in Example 6, and the reaction was investigated by changing the type of dehydrating agent. The reaction results are shown in Table 2.
[0070] Table 2 Influence of dehydrating agent types on the reaction
[0071]
[0072]
[0073] In Examples 11-16, 2,3,4,5-tetrachlorobenzonitrile was prepared u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com