Phenalide hydrazide compound as well as preparation method and application thereof
A technology of phthalide hydrazides and compounds, which is applied in the field of phthalide hydrazide compounds and their preparation, and can solve problems such as gaps in antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of 1-butyl-3-oxo-1,3-dihydroisobenzofuran-5-carbonitrile (formula III)
[0052] Under nitrogen protection, compound of formula II, 6-bromo-3-butylisobenzofuran-1(3H)-one (2.5 g, 9.3 mmol) and cuprous cyanide (2.0 g, 14 mmol) were dissolved in N-methyl pyrrolidine (NMP, 10 mL), and reacted at 180 °C for 3 h. After the reaction was completed, the reaction solution was cooled to room temperature, 50 mL of water was added, extracted with ethyl acetate (50 mL × 3), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. After recrystallization from ethyl acetate, 1.0 g of a white solid compound of formula III was obtained in a yield of 50.0%, m.p. 82-83°C. 1 HNMR (600MHz, CDCl 3 )δ8.19(d,J=1.2Hz,1H),7.95(dd,J=8.4,1.2Hz,1H),7.61(d,J=7.8Hz,1H),5.56-5.54(m,1H), 2.11-2.05(m, 1H), 1.82-1.76(m, 1H), 1.52-1.36(m, 4H), 0.93(t, J=7.2Hz, 3H); 13 C NMR (150MHz, CDCl 3 )δ168.2,153.8,137.0,129.9,127.5,123.1,117.3,113.7,81.5,34.1,26.8,22.3,13.8; HRMS(...
Embodiment 2
[0054] Preparation of 1-butyl-3-oxo-1,3-dihydroisobenzofuran-5-carboxylic acid (formula IV)
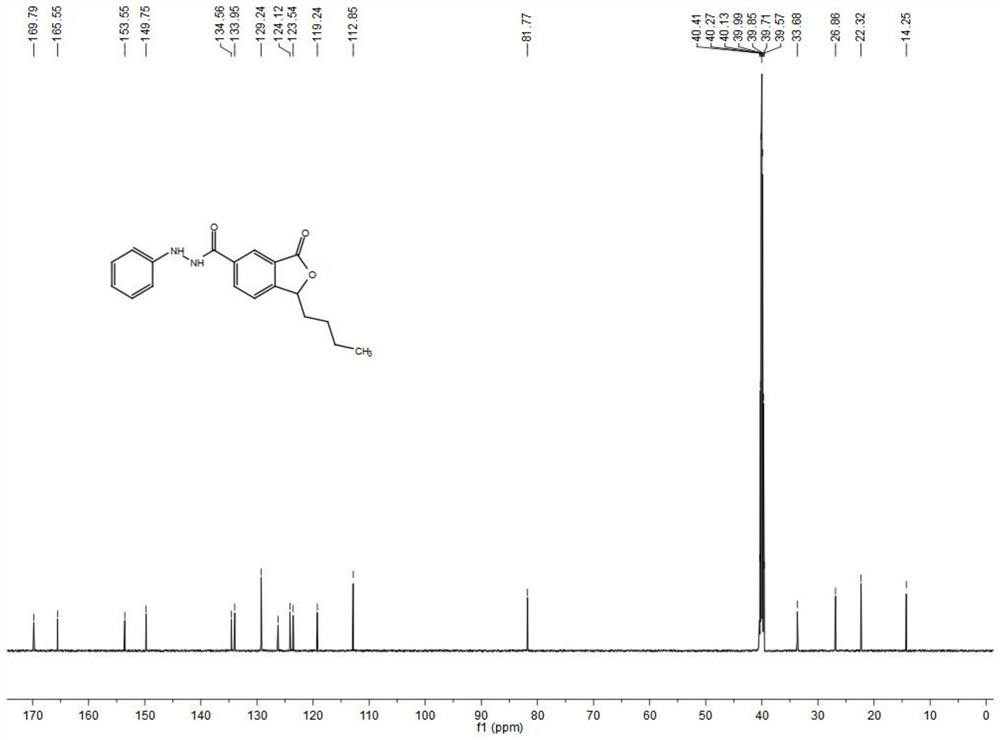
[0055] Under nitrogen protection, compound formula III (1.0 g, 4.65 mmol) was dissolved in 98% H 2 SO 4 (2.5mL), add H at 0°C 2 O (2.5mL), stirred at room temperature for 0.5h, then moved to 100°C oil bath for reflux reaction for 24h, after the reaction was complete, poured the reaction solution into 20mL of water, extracted with ethyl acetate (50mL×3), dried over anhydrous sodium sulfate, reduced After being concentrated under pressure, recrystallized from ethyl acetate to obtain 0.71 g of a white solid compound of formula IV, yield 65.1%, m.p. 145-147°C. 1 H NMR (600MHz, DMSO-d 6 )δ9.10(d,J=7.8Hz,1H),9.04(s,1H),8.62(d,J=7.8Hz,1H),6.53-6.51(m,1H),2.96-2.86(m,1H) ), 2.54-2.49(m, 1H), 2.14-2.03(m, 4H), 1.66(t, J=6.6Hz, 3H); 13 C NMR (150MHz, DMSO-d 6 )δ169.5,166.6,154.6,135.3,132.5,126.4,126.0,123.6,81.8,33.6,26.9,22.3,14.2;HRMS(ESI)calcd for C 13 H 15 O 4 [M+H] + m / z: 235.09...
Embodiment 3
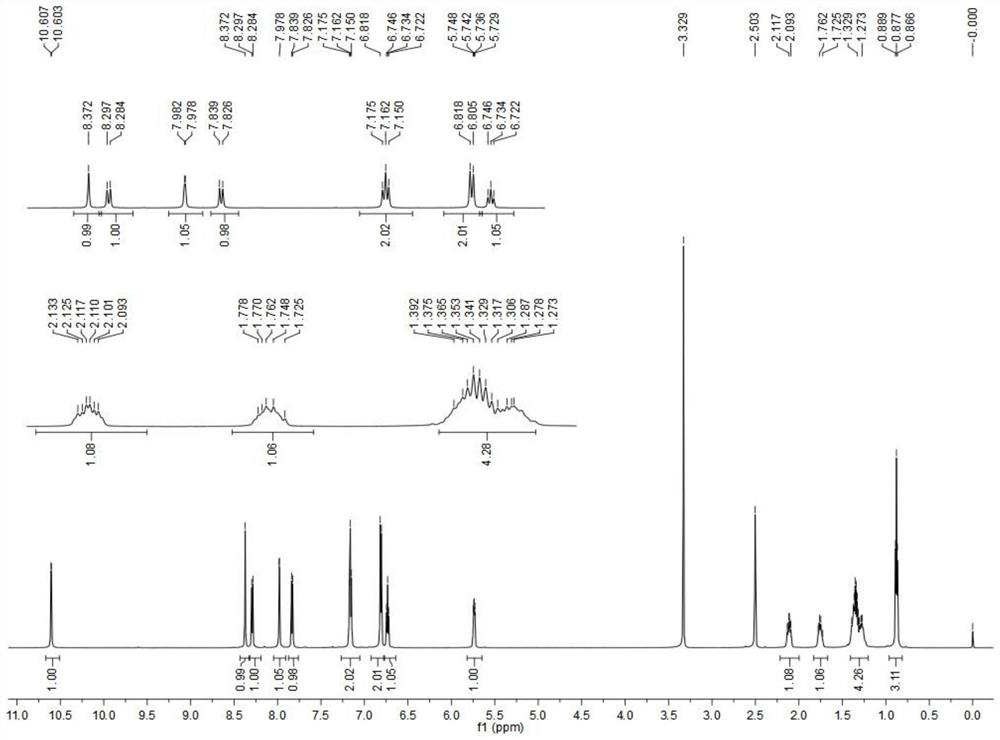
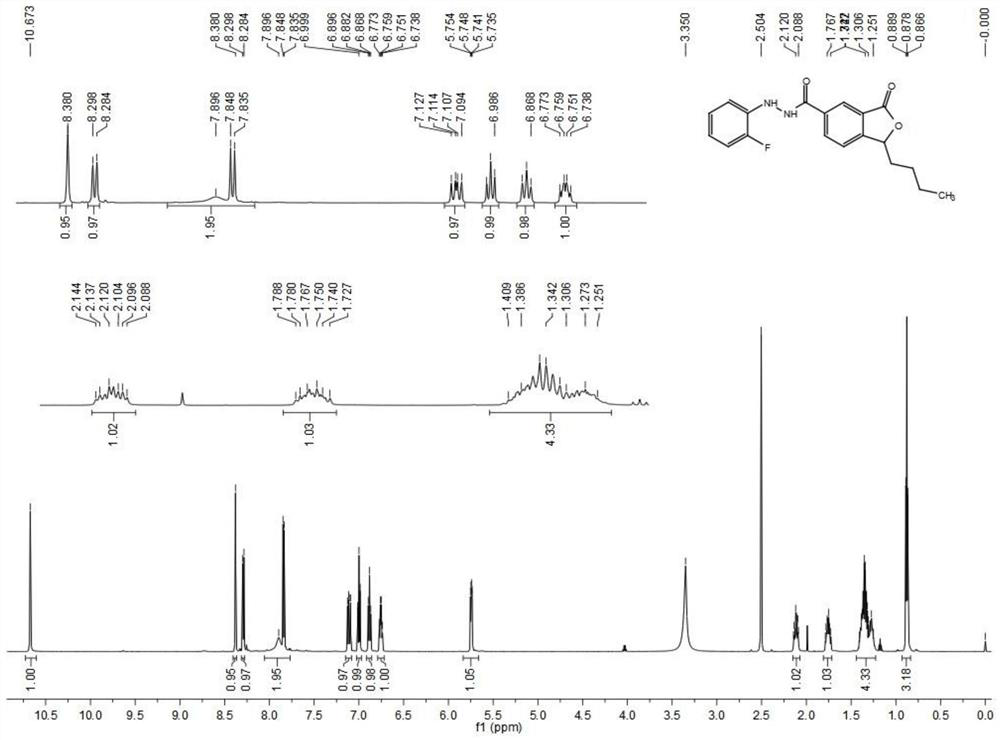
[0057] Preparation of arylhydrazinephthalide compounds (formula V):
[0058] Under nitrogen protection, the compound of formula IV (80 mg, 0.34 mmol) was dissolved in thionyl chloride (2 mL), and the reaction was refluxed for 8 h. TLC detected that the reaction was completed. The reaction solution was concentrated and dissolved in dry tetrahydrofuran for later use; another In a 10 mL double-necked flask, the aryl hydrazine hydrochloride compound (0.2 mmol) was dissolved in dry pyridine (2 mL), slowly dropped into the above tetrahydrofuran solution at 0°C, and the reaction was carried out at room temperature for 1 h. TLC detected the completion of the reaction. It was poured into 20 mL of water, extracted with dichloromethane (20 mL×3), dried over anhydrous sodium sulfate, concentrated and recrystallized from ethyl acetate to obtain compound formula V. Specific compounds are shown in Table 1.
[0059] Table 1 When R 1 When it is butyl, the specific compound formula V
[0060...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com