Borate chemotherapy sensitizer with symmetrical structure as well as preparation method and application of borate chemotherapy sensitizer
A symmetrical structure and sensitizer technology, applied in chemical instruments and methods, active ingredients of boron compounds, medical preparations containing active ingredients, etc., can solve problems such as limited practical application, achieve easy modification or reaction, and simple preparation method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of chemosensitizer 2BOH with symmetrical structure:
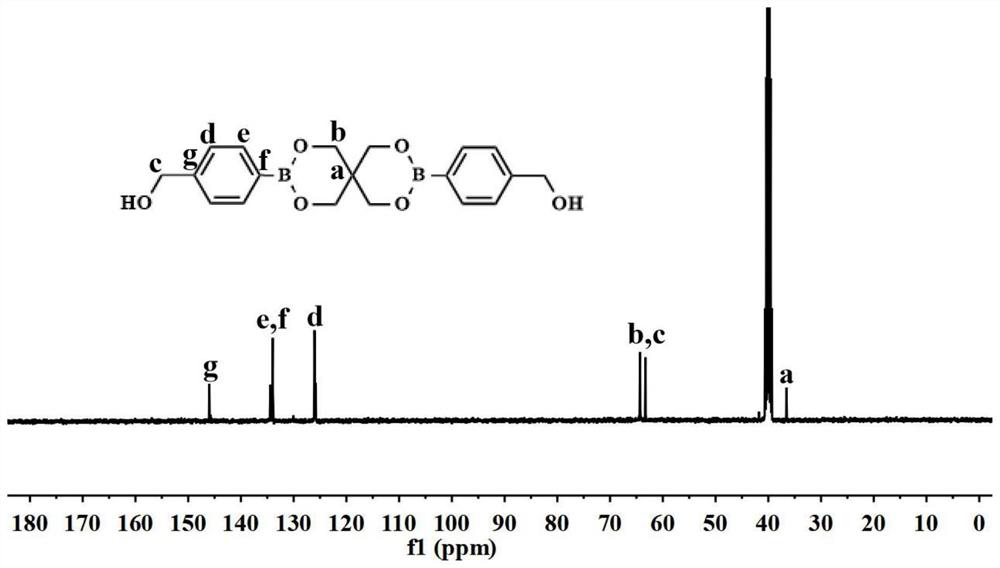
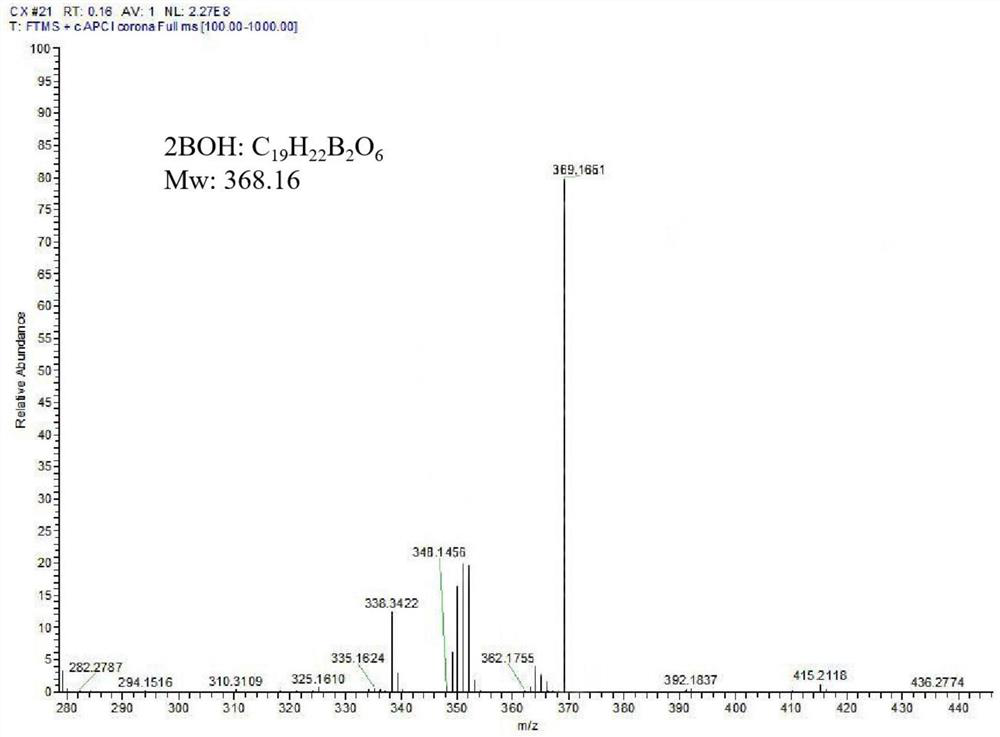
[0035] Weigh pentaerythritol, 2-hydroxymethylphenylboronic acid, and water-binding agent in a 100-ml reaction flask at a molar ratio of 1:2.5:8, and use anhydrous tetrahydrofuran as a solvent. At room temperature, it was stirred slowly with nitrogen gas for 24 hours; after the reaction, it was filtered twice with a sand core funnel by natural dripping method, the filtrate was evaporated to dryness, and then washed with an aqueous sodium hydroxide solution with a pH of 8.0. The silica gel column was separated and purified and then frozen. After drying, a white powdery product with a symmetrical structure was obtained as a chemosensitizer named 2BOH, and the yield was 62.16%;
[0036] Its reaction equation is as follows:
[0037]
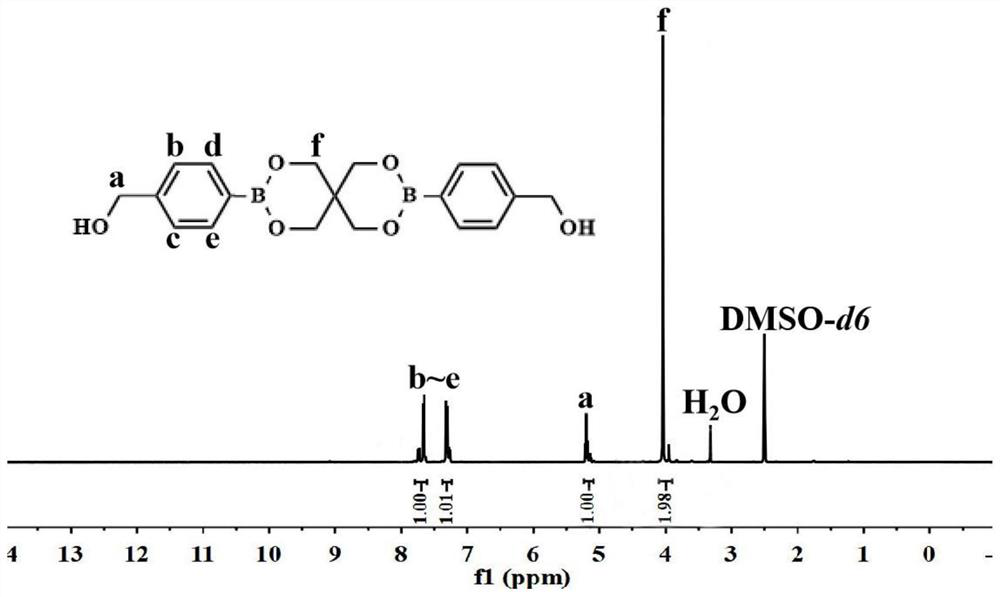
[0038] Chemosensitizer 2BOH 1 H NMR as figure 1 shown: 1 H NMR (400MHz, DMSO-d6, δ, ppm): 4.04 (s, 8H, -O-CH2-C), 5.20 (d, 4H, -CH2-Ar), 7.27-7.57 (m, 8H, -ARH ).
[0039]...
Embodiment 2
[0042] The effect of 2BOH on intracellular GSH levels:
[0043] Human lung cancer cells (A549) or human lung cancer cisplatin-resistant cells (A549 / DDP) were added to a six-well plate of cells and cultured overnight to allow cells to adhere. Then, the old medium was aspirated, and 1.8 mL of fresh medium and 200 μL of 2BOH at various concentrations were added to each well. The culture was continued for 4 h, then the cells were washed with PBS, the cell structure was disrupted with lysis buffer, and the supernatant was collected by centrifugation at 2000 rpm. Finally, the GSH content in the supernatant was determined using the GSH / GSSG kit.
[0044] The result is as Figure 4As shown, the GSH level in cisplatin-resistant human lung cancer cells is much higher than that in human lung cancer cells, that is, the GSH concentration in drug-resistant tumor cells is higher than that in drug-sensitive tumor cells; when treated with different concentrations of 2BOH, the intracellular G...
Embodiment 3
[0046] Cytotoxicity assay of 2BOH:
[0047] Human lung cancer cells (A549) or human lung cancer cisplatin-resistant cells (A549 / DDP) were seeded in a 96-well plate with about 5,000 cells per well. After overnight culture, the old medium was removed and 180 μL of fresh medium was added. , 20 μL of different concentrations of 2BOH (the concentration was set at 5-160 μg / mL), and continued to co-culture for 24 h. Finally, the medium was removed, 150 μL of DMSO was added, and after shaking for 10 min, the absorbance of crystal violet produced by living cells was detected at a wavelength of 570 nm, and the cell viability was calculated.
[0048] The result is as Figure 5 As shown, in A549 and A549 / DDP cells, cell viability was generally higher after 2BOH treatment, and only at higher 2BOH concentrations, showed weaker cytotoxicity. This is due to the massive consumption of intracellular GSH by 2BOH, resulting in a redox imbalance, which in turn triggers reactive oxygen species to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com