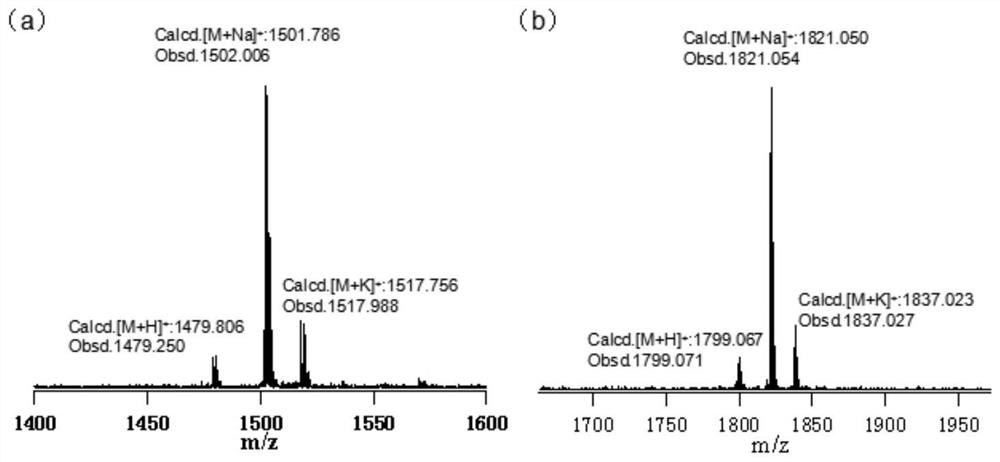
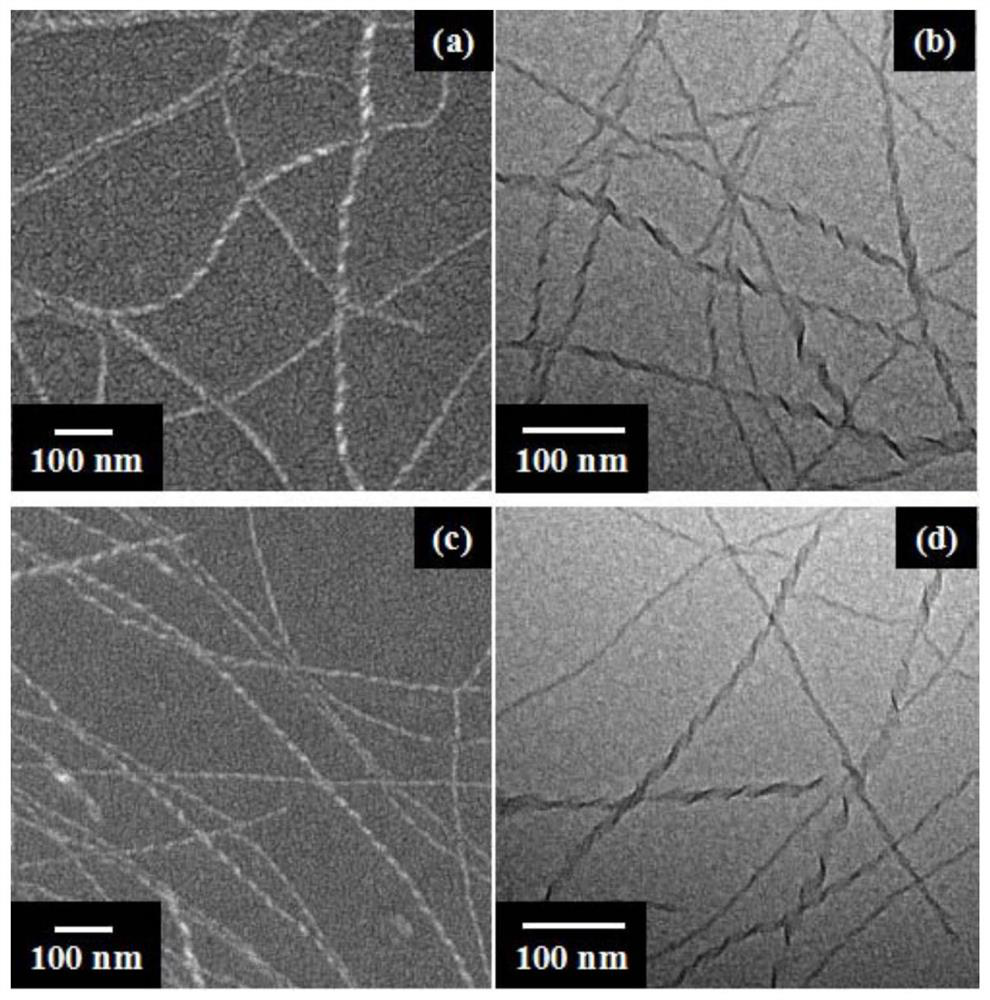
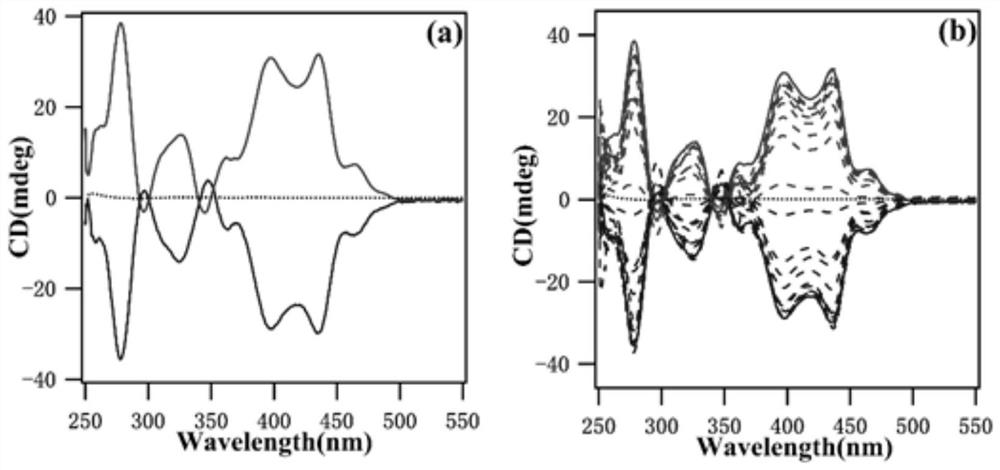
A kind of hbc compound containing crown ether and its preparation and application
A compound and crown ether-containing technology, which are applied to supramolecular assembly materials and their preparation and application fields, can solve problems such as reduced efficiency and difficulty, and achieve the effects of simple synthesis method, good solubility and mature preparation technology of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of Compound 4
[0040] Add 3 (400mg, 1.03mmol), bispinacol diboron (313mg, 1.23mmol), PdCl 2 (dppf) (75mg, 0.103mmol, 10%), KOAc (221mg, 2.25mmol, 3eq.), pumped and exchanged air three times under the protection of Ar gas, added anhydrous DMF (freezing deoxygenation) (20mL), heated to 80°C , reacted for 3 hours, and stopped the reaction. After cooling to room temperature, CH 2 Cl 2 Extract (50mL×3), combine the organic phases, wash with distilled water (50mL×3), dry the organic phase with anhydrous magnesium sulfate, filter to remove the magnesium sulfate, rotary evaporate to remove the solvent, and the residue is purified by silica gel chromatography (eluent : dichloromethane: methanol = 20: 1), to obtain light yellow oil: 200.41mg, yield 61%. 1 H NMR (600MHz, CDCl 3 )δ (ppm) 7.08 (d, J = 7.1Hz, 2H), 6.93 (d, J = 8.4Hz, 1H), 4.23 (d, J = 20.9Hz, 4H), 3.98 (s, 4H), 3.81 ( s,4H),3.75(s,4H),3.72(s,4H),1.27(s,12H). 13 C NMR (150MHz, CDCl 3 ): δ (ppm) 148....
Embodiment 2
[0042] Synthesis of compound 6
[0043] Add compound 5 (5g, 15.36mmol), cuprous iodide (0.293g, 1.54mmol), PdCl 2 (PPh 3 ) 2 (0.66g, 0.9mmol), under the protection of Ar, gas exchanged three times, added DBU (14.24mL), benzene (20mL), trimethylethynyl silicon (TMSA, 1.1mL) and H 2 O (60 μL), heated to 80°C, stopped the reaction after 24 hours and cooled to room temperature. CH 2 Cl 2 Take three times (30mL×3), combine the organic phase, wash the organic phase with 10% HCl, then wash with saturated NaCl, rotary evaporate most of the solvent, add an appropriate amount of silica gel for dry column chromatography, (eluent: petroleum ether) to obtain 2.1 g of a colorless transparent liquid with a yield of 65%. 1 H NMR (600MHz, CDCl 3 ): δ (ppm) 7.45 (d, J = 7.3Hz, 4H), 7.17 (d, J = 7.2Hz, 4H), 2.62 (d, J = 6.6Hz, 4H), 1.62 (s, 4H), 1.30 (d, J = 17.1 Hz, 36H), 0.90 (d, J = 6.5 Hz, 6H). 13 C NMR (150MHz, CDCl 3 ): δ (ppm) 143.6, 131.8, 128.8, 121.0, 89.3, 36.3, 36.2, 31.6, 30...
Embodiment 3
[0045] Synthesis of compound 7
[0046] Add 6 (2.46g, 4.79mmol) to the 500mL two-necked flask, RuO 2 (14mg), add CH3CN / CCl4 / H2O=14mL / 14mL / 21mL in proportion, stir at 25°C for 2.5 hours, distill off the solvent, and use CH 2 Cl 2 Extract (50mL×3), combine the organic phase, dry the organic phase with anhydrous magnesium sulfate, filter to remove the magnesium sulfate, rotary evaporate to remove the solvent, and the residue is purified by silica gel chromatography, (eluent: petroleum ether: dichloromethane =3:1), 1.6 g of a light yellow solid was obtained, and the yield was 85%. 1 H NMR (600MHz, CDCl 3 ): δ (ppm) 7.91 (d, J = 7.9Hz, 4H), 7.33 (d, J = 8.0Hz, 4H), 2.70 (t, J = 7.7Hz, 4H), 1.65 (m, 4H), 1.30 (d, J=18.0Hz, 36H), 0.90 (t, J=6.6Hz, 6H). 13 C NMR (150MHz, CDCl 3 ): δ (ppm) 194.8, 151.2, 131.2, 128.8, 129.3, 36.5, 32.2, 31.3, 29.9, 29.8, 29.7, 29.6, 29.5, 23.0, 14.4. MALDI–TOF–MS: Calcd.for C 38 h 58 o 2 [M+H] + :m / z=546.88; Found: 546.98.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com