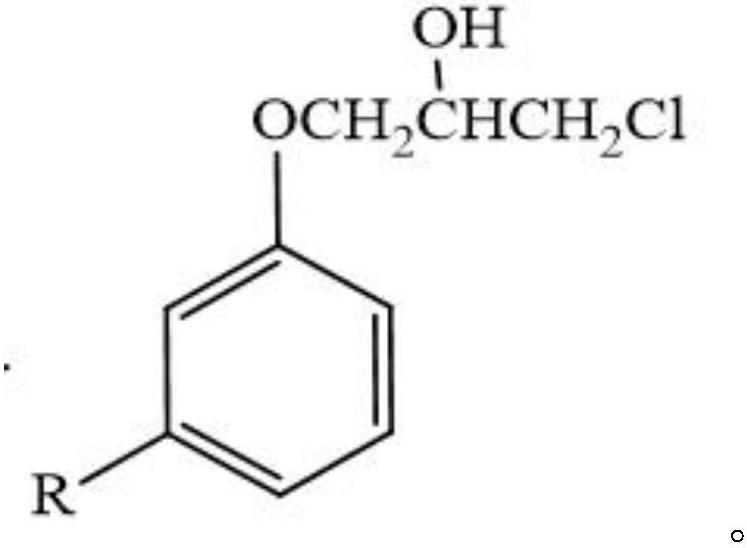
Preparation method and application of cardanol-based chlorohydrin ether
A cardanol-based chlorohydrin technology and cardanol technology are applied in the preparation field of cardanol-based chlorohydrin, can solve the problems of poor surfactant performance, long synthesis route, low yield and the like, and achieve high reaction yield, Simple preparation process and high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention discloses a preparation method of cardanol-based chlorohydrin, comprising the following steps:
[0024] S1, add cardanol, epichlorohydrin and organic alcohol in the reactor, stir and dissolve, wherein the mol ratio of cardanol and epichlorohydrin is 1: 1~1: 8, and the mol ratio of cardanol and organic alcohol is 1:10~1:80;
[0025] S2, adding an organic alcohol solution containing a phase transfer catalyst into the reactor, and performing constant temperature reaction at 60-100° C. for 4-10 hours;
[0026] S3, removing organic alcohol and unreacted epichlorohydrin after completion of the reaction, washing the product, and removing the phase transfer catalyst to obtain cardanol-based chlorohydrin.
[0027] In one embodiment, the cardanol is technical grade unsaturated cardanol.
[0028] In one embodiment, the organic alcohol of the present invention is a short-chain alcohol; in another embodiment, the short-chain alcohol of the present invention is one or...
Embodiment 1
[0044] Preparation of Surfactant Cardanol-Based Chlorohydrin:
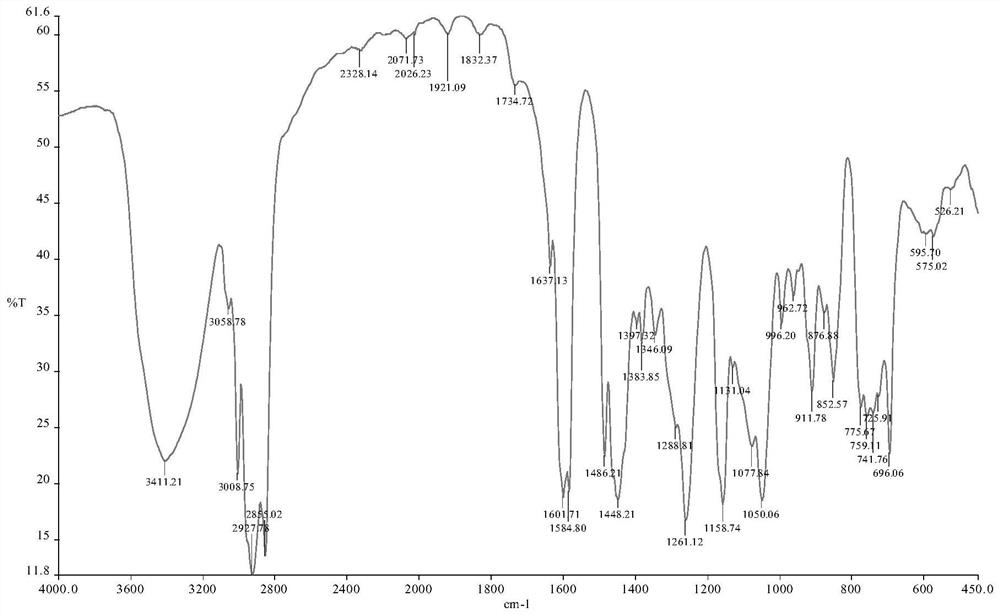
[0045] Add 5.80g (0.02mol) technical grade unsaturated cardanol, 3.70g (0.04mol) epichlorohydrin and 80mL ethanol to the reactor, stir and dissolve at room temperature; then add 20mL mass fraction of 3% tetrabutyl bromide The ethanol solution of ammonium chloride was reacted at a constant temperature of 80 ° C for 6 hours; after the reaction was completed, the solvent and unreacted epichlorohydrin were removed by distillation under reduced pressure, the distilled water was used to wash the distillation product for many times, and tetrabutylammonium bromide was removed to obtain cashew nuts. Phenol chlorohydrin ether, the yield was 95.3%, and the purity was 98.2%. Infrared spectrum of cardanol chlorohydrin see figure 1 , as can be seen from the figure, the wave number is 3411cm -1 The left and right are the O-H stretching vibration absorption peaks in the alcoholic hydroxyl group; 2927cm -1 , 2886cm -1 Left and...
Embodiment 2
[0047] Add 5.80g (0.02mol) technical grade unsaturated cardanol, 11.10g (0.12mol) epichlorohydrin and 80mL isopropanol to the reactor, stir and dissolve at room temperature; then add 20mL mass fraction of 2% tetradecanol The isopropanol solution of alkyl trimethyl ammonium chloride was reacted at a constant temperature of 80 ° C for 8 hours; after the reaction was completed, the solvent and unreacted epichlorohydrin were removed by distillation under reduced pressure, and the distilled water was used to wash the distillation product several times to remove ten Tetraalkyltrimethylammonium chloride was obtained to obtain cardanol chlorohydrins with a yield of 91.5% and a purity of 97.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com