Method for producing lithium carbonate by leaching spodumene with nitric acid
A technology of lithium carbonate and nitric acid, applied in the direction of lithium carbonate;/acid carbonate, process efficiency improvement, etc., can solve the problems of low comprehensive utilization rate of auxiliary materials, low lithium recovery rate, and inability to recycle, etc. To achieve the effect of increasing the comprehensive utilization rate, being environmentally friendly and improving the complex process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
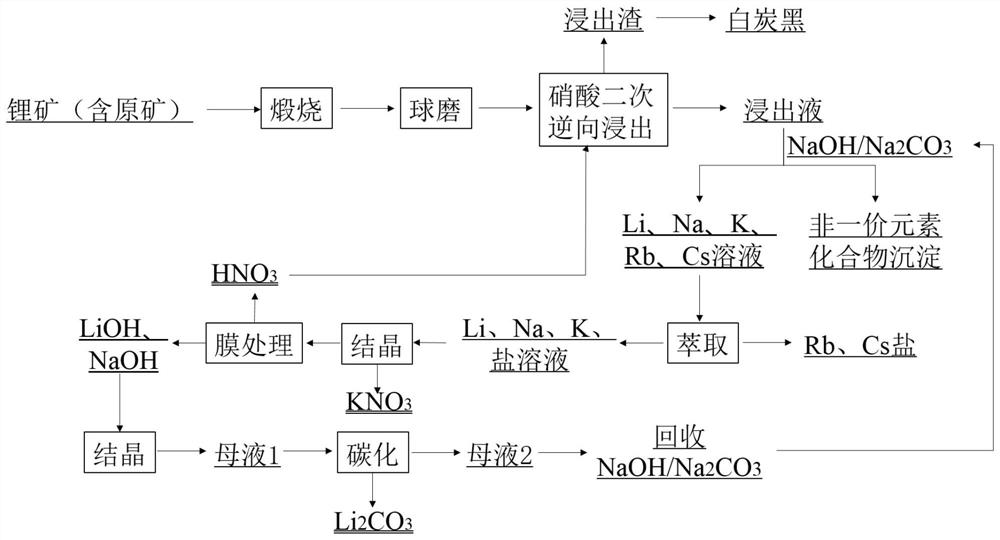
[0049] like figure 1 Shown, is the process flow diagram of the method for producing lithium carbonate by leaching spodumene nitric acid of the present invention; the detailed steps of the method for producing lithium carbonate by leaching spodumene nitric acid of the present invention are as follows:
[0050] A method for producing lithium carbonate by leaching spodumene (containing raw ore) with nitric acid specifically comprises the following steps:
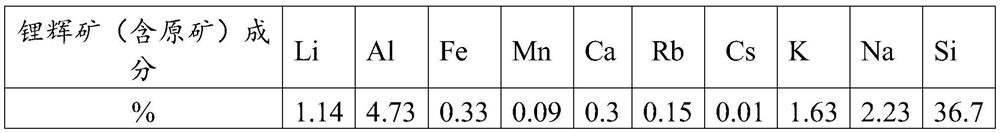
[0051] Step 1: calcining spodumene (containing raw ore) at 1000° C. for 3 hours, and ball-milling the spodumene (containing raw ore) after calcination, and the average particle size of spodumene (containing raw ore) after ball milling is less than 48 μm.
[0052] Step 2: Slurry the pretreated spodumene (containing raw ore) in step 1 and water at a liquid-solid mass ratio of 2.5:1, and the slurry is subjected to secondary reverse leaching of nitric acid. 120wt%, the amount of nitric acid used in the secondary leaching of nitric a...
Embodiment 2
[0059] A method of leaching spodumene (containing raw ore) with nitric acid to produce lithium carbonate is implemented by the method described in Example 1, and the difference is:
[0060] In the step 1, the calcination temperature is 1050°C, and the calcination time is 2h.
[0061] Step 2: Slurry the pretreated spodumene (containing raw ore) in step 1 and water at a liquid-solid mass ratio of 3:1, and the slurry is subjected to secondary reverse leaching of nitric acid, wherein the amount of nitric acid in the primary leaching of nitric acid is the theoretical amount. 150wt%, the amount of nitric acid in the secondary leaching of nitric acid is 30wt% of the theoretical amount, the reaction temperature of the primary leaching and the reaction temperature of the secondary leaching are 150 ℃, and the reaction time of the primary leaching and the secondary leaching are 3h respectively.
[0062] In the described step 4, the extractant used is 4-tert-butyl-2-(α-methylbenzyl)phenol...
Embodiment 3
[0067] A method of leaching spodumene (containing raw ore) with nitric acid to produce lithium carbonate is implemented by the method described in Example 1, and the difference is:
[0068] In the step 1, the calcination temperature is 1100°C, and the calcination time is 1.5h.
[0069] Step 2: Slurry the pretreated spodumene (containing raw ore) in step 1 and water at a liquid-solid mass ratio of 3:1, and the slurry is subjected to secondary reverse leaching of nitric acid, wherein the amount of nitric acid in the primary leaching of nitric acid is the theoretical amount. 145%, the amount of nitric acid in the secondary leaching of nitric acid is 40% of the theoretical amount, the reaction temperature of the primary leaching and the reaction temperature of the secondary leaching are 170 °C, and the reaction time of the primary leaching and the secondary leaching are 2.5h respectively.
[0070] In the step 5, the crystallization temperature is 30°C.
[0071] The Li finally pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com