Flower-shaped structure photocatalyst for reducing carbon dioxide as well as preparation method and application of flower-shaped structure photocatalyst
A carbon dioxide and flower-like structure technology, applied in the field of photocatalysis, can solve the problems of low carrier mobility, preparation process load, and low catalytic efficiency, and achieve the effects of simple operation, low cost, and low working temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 0.233 g of cobalt nitrate, 0.15 g of aluminum nitrate, 0.15 g of ammonium fluoride and 1.7 g of urea were dissolved in 40 mL of deionized water and magnetically stirred at room temperature for one hour. The precursor was transferred to a 100-ml polytetrafluoroethylene stainless steel autoclave, and then placed in a constant temperature drying oven, sealed and heated at 90°C for 8 hours. After natural cooling to room temperature, rinse thoroughly with deionized water and ethanol. The obtained powder was dried in air at 60 °C for 12 h and named CoAl-LDHs.
[0036] Figure 7 show that the CH of the uncalcined precursor CoAl-LDHs catalyst 4and CO yields were 40.37 μmol / g and 20.24 μmol / g, respectively.
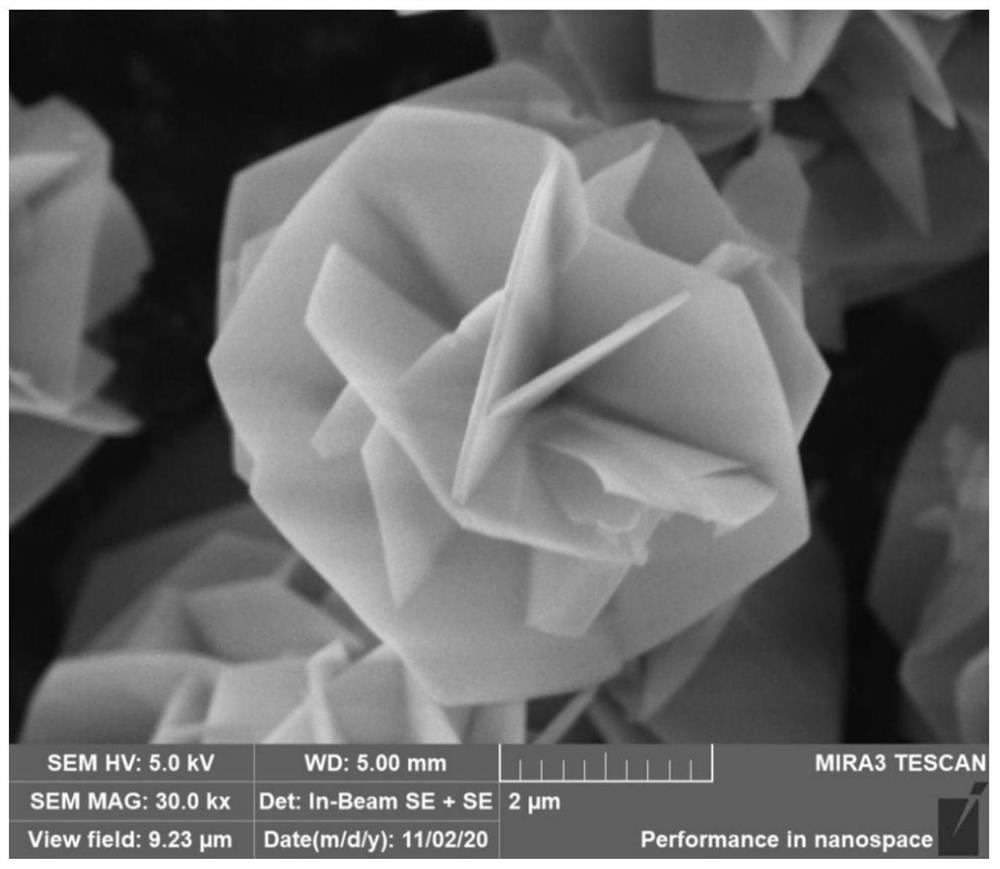
[0037] The obtained precursor powder was placed in a porcelain crucible under an air atmosphere at 5 °C min -1 The heating rate was heated in a muffle furnace, and calcined to 650 °C for 3 h, and the obtained product was named CoAl-650. The scanning electron microscope...
Embodiment 2
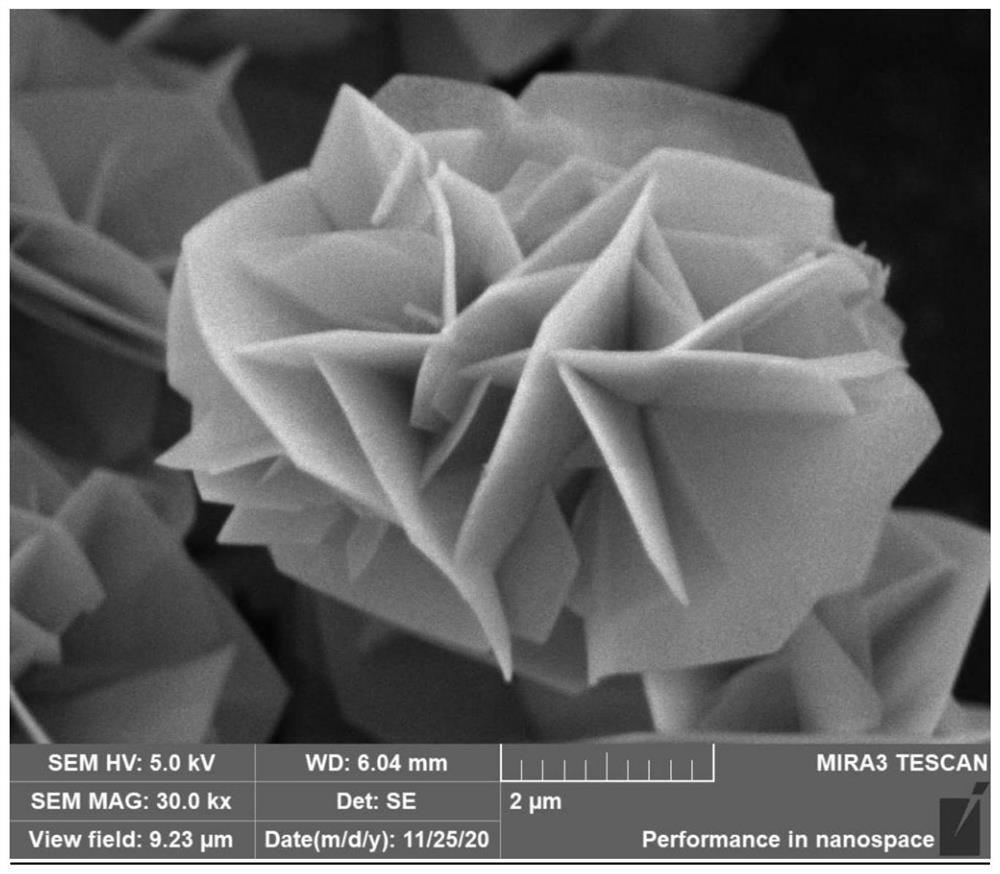
[0040] The method used in this example is the same as that used in Example 1, and the only difference is that the calcination procedure is different. In this example, the calcination was performed to 750° C., and the obtained product was named CoAl-750. The scanning electron microscope image of the thinner 3D flower-like CoAl-LDHs precursor obtained in Example 2 at 750 °C is as follows image 3 shown. Figure 4 The TEM image of the thinner 3D flower-like CoAl-LDHs precursor obtained in Example 2 calcined at 750°C. Figure 5 It is the X-ray diffraction pattern of the thin 3D flower-like CoAl-LDHs precursor obtained in Example 2 calcined at 750 ° C of the composite material; it can be seen from the figure that the ultrathin 3D porous CoAl 2 O 4 A composite material is formed. Example 2 to obtain 3D porous flower-like CoAl 2 O 4 The composites were irradiated with CO and CH under visible light at 200-800 nm for 7 hours 4 A comparison of yields such as Figure 7 shown.
[...
Embodiment 3
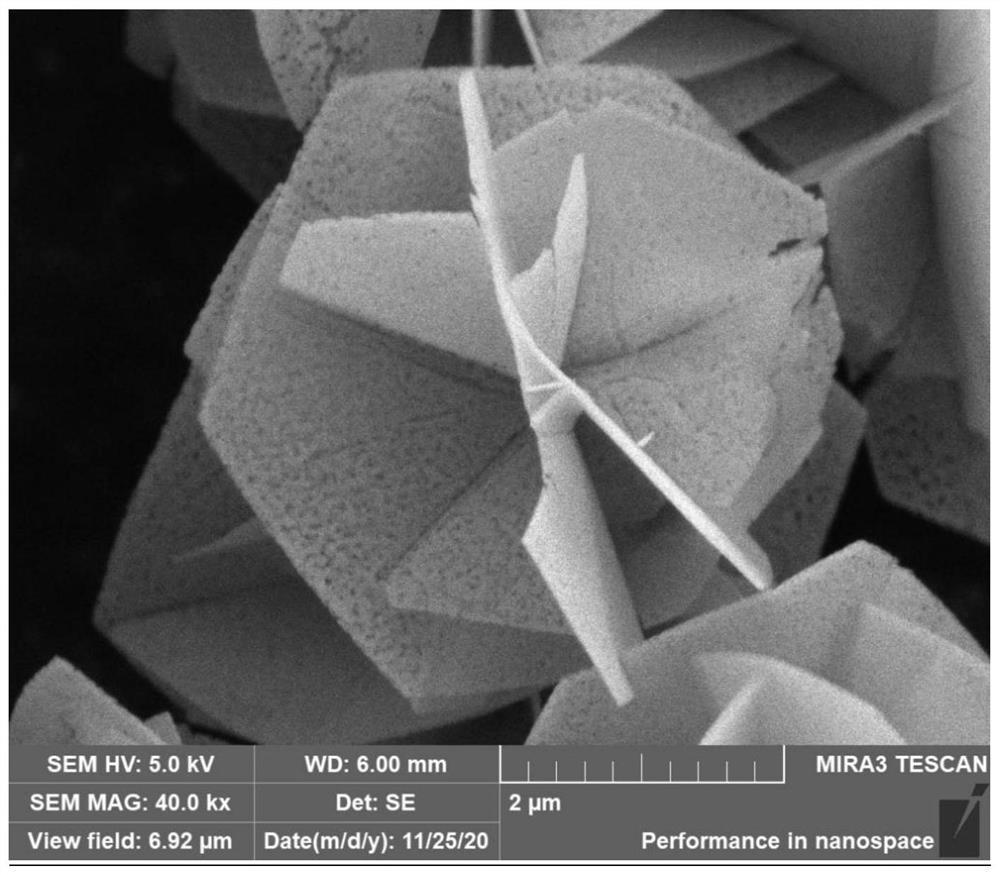
[0043] The method used in this example is the same as that used in Example 1, and the difference is only in the calcination procedure. In this example, the calcination was carried out to 850° C., and the obtained product was named CoAl-850. The scanning electron microscope image of the thinner 3D flower-like CoAl-LDHs precursor obtained in Example 3 calcined at 850 °C is as follows Image 6 shown. Example 3 to obtain 3D porous flower-like CoAl 2 O 4 The composites were irradiated with CO and CH under visible light at 200-800 nm for 7 hours 4 A comparison of yields such as Figure 7 shown.
[0044] Figure 7 shows that the 3D porous flower-like CoAl obtained by calcination at 850 °C 2 O 4 Catalyst CH 4 and CO yields were 46.12 μmol / g and 25.29 μmol / g, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com