Synthesis method of 3, 3, 5-trimethyl cyclohexanol
A technology of trimethylcyclohexanol and its synthesis method, which is applied in the field of synthesis of 3,3,5-trimethylcyclohexanol, can solve the problems of product cost increase, waste lye environmental pollution, etc., and avoid post-processing , Improve the hydrogenation activity and simplify the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
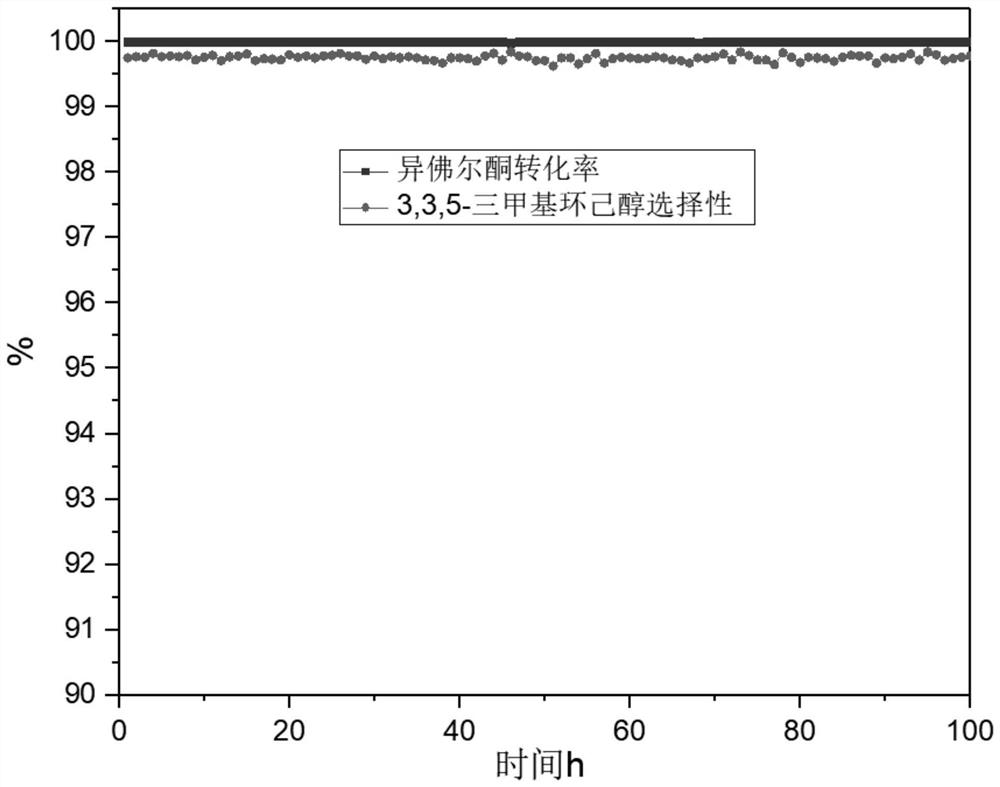
[0035] In a 10ml fixed bed reactor, the deep hydrogenation of isophorone to 3,3,5-trimethylcyclohexanol was carried out using a Zn-promoted Ni-Mo unsupported catalyst. The reaction temperature was 140 °C and the reaction pressure was 1.5 MPa. , the raw material liquid phase space velocity is 0.5h -1 , under the condition of hydrogen oil volume ratio of 240, through gas chromatography analysis, the conversion rate of raw material isophorone is 99.46%, the average selectivity of 3,3,5-trimethylcyclohexanol is 99.78%, and the ratio of cis-trans isophorol is 99.78%. 5.8.
[0036] The used Zn-promoted Ni-Mo unsupported catalyst preparation steps:
[0037] (1) At 30°C, add 0.5 g of ethanolamine to 60 ml of deionized water, and stir evenly.
[0038] (2) Continue to add 15.0g basic nickel carbonate, 10.8g ammonium heptamolybdate and 1.6g zinc nitrate into the above solution, and stir for 1h.
[0039] (3) Pour the above mixture into the reaction kettle, crystallize at 150°C for 6h, co...
Embodiment approach 2
[0044] In a 10ml fixed bed reactor, the deep hydrogenation of isophorone to 3,3,5-trimethylcyclohexanol was carried out using a Zn-promoted Ni-Mo unsupported catalyst, the reaction temperature was 160 °C, and the reaction pressure was 2.0 MPa , the raw material liquid phase volume space velocity is 1.5h -1 , under the condition of hydrogen oil volume ratio of 240, through gas chromatography analysis, the conversion rate of raw material isophorone is 99.52%, the average selectivity of 3,3,5-trimethylcyclohexanol is 99.09%, and the ratio of cis-trans isophorol is 99.09%. 6.1.
[0045] The used Zn-promoted Ni-Mo unsupported catalyst preparation steps:
[0046] (1) Add 1.5 g of ethanolamine to 60 ml of deionized water under the condition of 45°C, and stir evenly.
[0047] (2) Continue to add 33.6 g of nickel acetate, 7.9 g of ammonium heptamolybdate, and 2.4 g of zinc nitrate into the above solution, and stir for 1.0 h.
[0048] (3) Pour the above mixture into the reaction kett...
Embodiment approach 3
[0053] In a 10ml fixed bed reactor, the deep hydrogenation of isophorone to 3,3,5-trimethylcyclohexanol was carried out using a zinc-promoted Ni-Mo unsupported catalyst. The reaction temperature was 160 °C and the reaction pressure was 2.0 MPa. , the raw material liquid phase space velocity is 1.5h -1 , under the condition of hydrogen oil volume ratio of 480, through gas chromatography analysis, the conversion rate of raw material isophorone is 99.81%, the average selectivity of 3,3,5-trimethylcyclohexanol is 99.54%, and the ratio of cis-trans isophorol is 99.54%. 6.3.
[0054] The used Zn-promoted Ni-Mo unsupported catalyst preparation steps:
[0055] (1) Add 1.9 g of ethanolamine to 60 ml of deionized water under the condition of 60°C, and stir evenly.
[0056] (2) Continue to add 26.2g of nickel nitrate, 14.1g of ammonium tetramolybdate and 3.2g of zinc nitrate into the above solution, and stir for 1h.
[0057] (3) Pour the above mixture into the reaction kettle, crystal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com