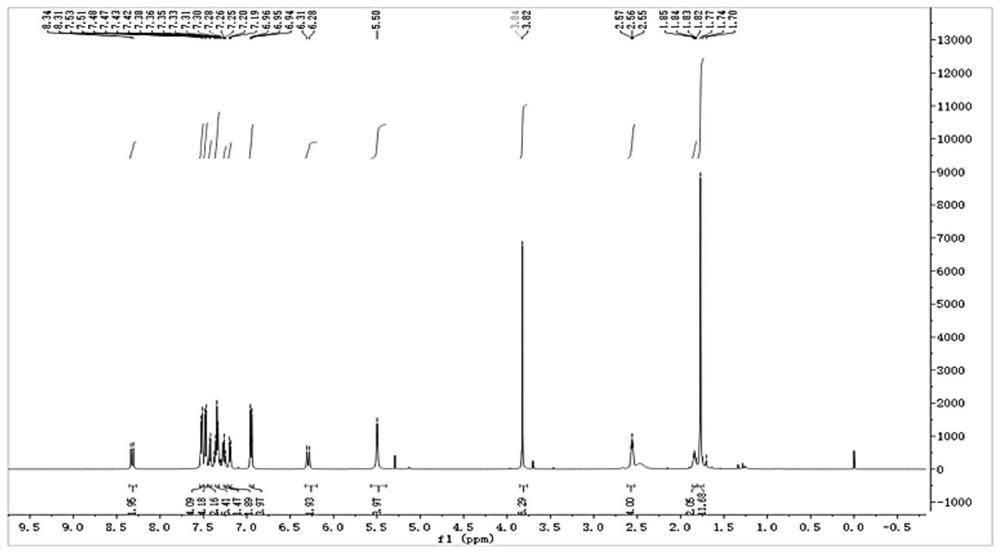
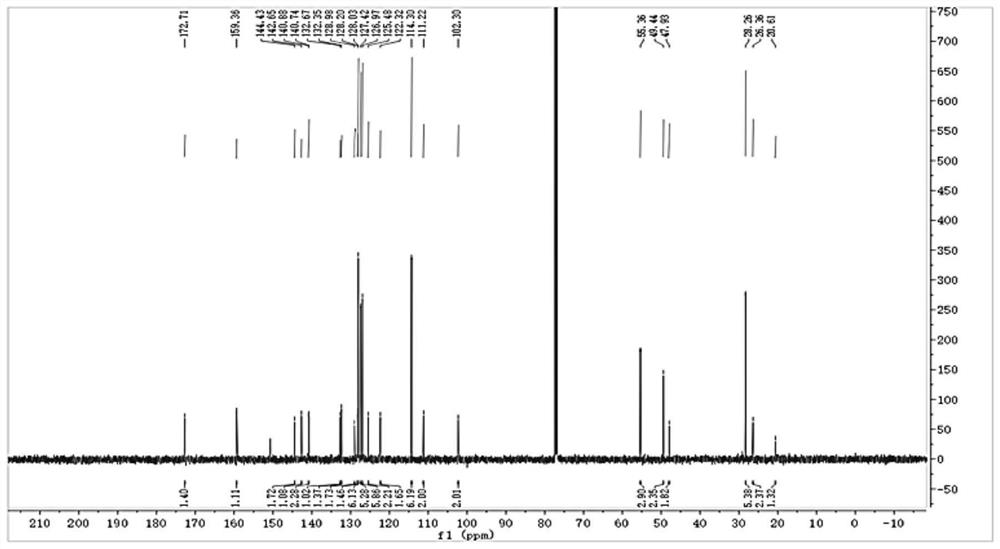
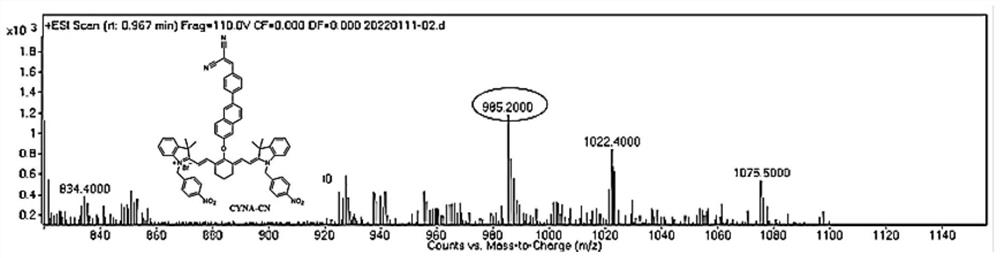
Near-infrared & two-photon dynamics dual-mode high-stability fluorescent dye, NTR&HClO probe and synthesis method and application
A fluorescent dye and two-photon technology, applied in the field of ROS&, can solve the problems of multiple wavelength coverage and fluorescence ratio dependence, and achieve the effect of enhancing electron transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The synthesis of a single-molecule dual-mode excitation probe (CYNA-CN for short) comprises the following steps:
[0062] 1. Synthesis of Y-n series compounds (refer to synthetic route 1): Benzyl bromide (2.59 g, 12 mmol) was dissolved in acetonitrile (20 mL deoxygenated in advance) under anaerobic operation, and heated at 85° C. in the dark until the solution became clear. 2,3,3-Trimethylindole (2.385 g, 16 mmol) was dissolved in deoxygenated acetonitrile (20 mL), then added dropwise to the above clear solution, and the reaction was continued for 12 h in the dark. After stopping the reaction, use a rotary evaporator to steam more than 70% of the solvent, then add the petroleum ether of anhydrous and anaerobic treatment to the solid product and separate out, and wash the solid product 3 times with the petroleum ether to obtain Y-n series compounds (n refers to the para-position). Benzyl bromide series derivatives with different substituents, as shown in Synthetic Scheme...
Embodiment 2
[0073]Near-infrared parent framework stability test: In order to select the most stable fluorescent dye as the parent structure, this embodiment uses the CY-n series compounds prepared in Example 1 to carry out time stability testing, specifically including: Light, no cooling) to analyze the chromatographic components of each CY-n dye (2 mM), and the sample solutions were all prepared with chromatographic grade acetonitrile. The samples were subjected to high performance liquid chromatography (HPLC) analysis at different time intervals (1h, 2h, 3h, 5h, 7h, 9h). HPLC adopts Agilent 1100 series high performance liquid chromatography (Waldbronn, Germany), mobile phase A: organic phase with 100% acetonitrile; mobile phase B: aqueous phase with 5% acetonitrile (containing 0.1% formic acid). Elution gradient: 0min=100%A, 3min=98%A, 10min=97%A, 15min=0%A. The gradient elution flow rate was 1 mL / min, and the injection volume was 10 μL.
[0074] The results are attached Image 6 sho...
Embodiment 3
[0076] The effect of pH on the fluorescence intensity of fluorescent dyes (CY-n series compounds): In order to select the most stable dye as the parent ring, in this example, the CY-n series compounds prepared in Example 1 were used to detect the effect of pH, which specifically included : To prepare PBS buffer solutions of different pH, each CY-n was prepared to 10 μM with buffer solutions of different pH (pH=3.0, 4.0, 5.0, 6.0, 6.8, 7.4, 8.0, 9.0), and then subjected to spectral test.
[0077] as attached Figure 7 As shown, the more stable dyes CY-1, CY-6, CY-7 and CY-8 were placed in the attached Figure 9 In a, that is, the dye whose fluorescence intensity is less affected by pH is the dye containing benzene ring, bromine, phenylboronic acid and nitro group connected to the N-position of cyanine; Figure 9 As can be seen in b, the other probes are relatively more affected by pH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com