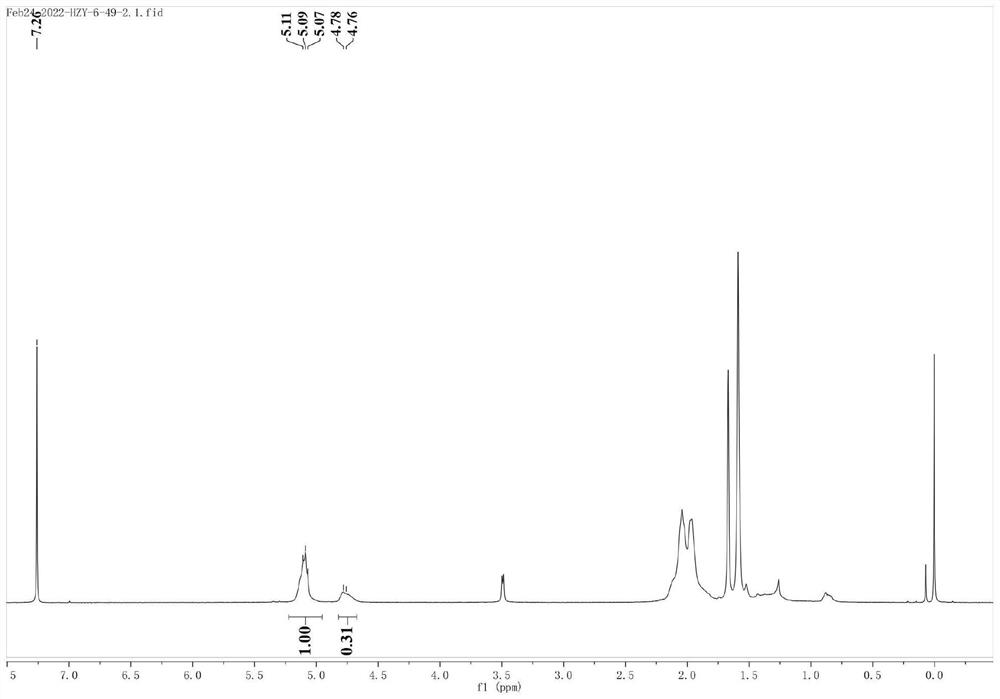
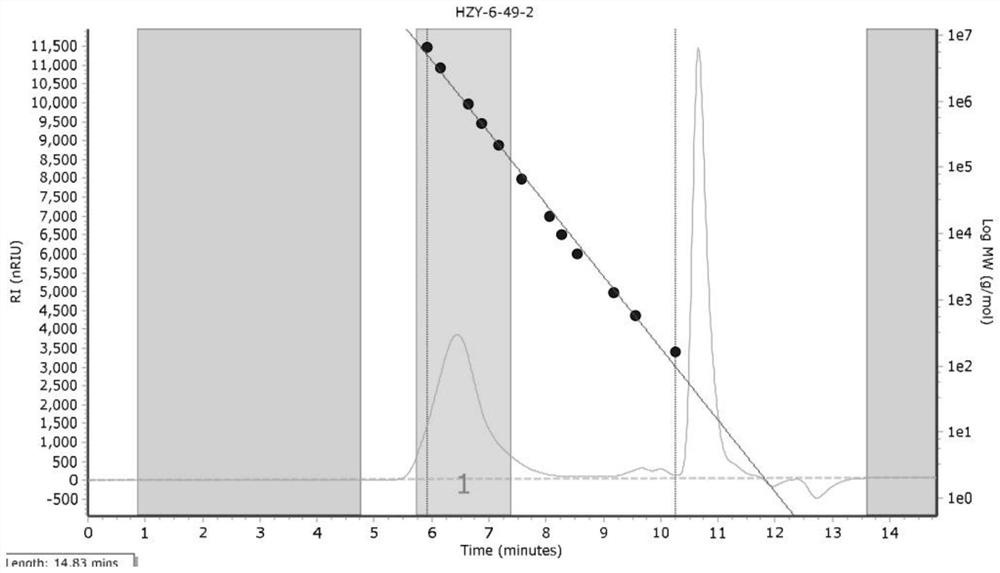
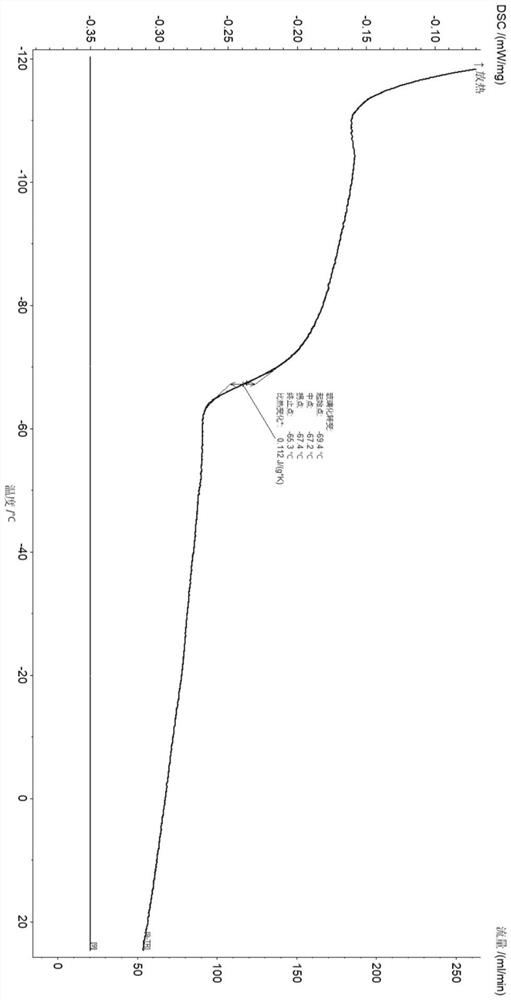
Pyridine imine oxime iron catalyst, preparation method thereof and application of pyridine imine oxime iron catalyst in conjugated diene polymerization
A technology of pyridine imidoxime and iron catalyst, which is applied in the direction of iron organic compounds and chemical recovery, can solve the problems of high production cost and low molecular weight, and achieve the effects of low price, simple preparation and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation method of a kind of pyridine imidoxime iron catalyst of the present embodiment is carried out according to the following steps:
[0027] Under an argon atmosphere, first add anhydrous FeCl to a 25 mL Schlenk tube 2 (1.27 mg, 10 μmol, 1 equiv.), then the pyridine imine ligand L1 (2.72 mg, 20 μmol, 2 equiv.) was added to the system, and then 20 mL of dichloromethane solvent was added, and the reaction was stirred at 25 ° C for 24 h, the reaction was completed After that, the filtrate was collected by filtration, concentrated to solid, washed with anhydrous n-hexane for 3 times, and dried in vacuum for 12 h to obtain iron pyridine imidoxime catalyst A (referred to as main catalyst A).
[0028] Mass spectrometry: C 14 H 16 Cl 2 FeN 4 O 2 : [M-Cl - ] + : theoretical value 363.0311, actual value: 363.0421.
[0029] Elemental analysis: Theoretical value: C, 42.14%; H, 4.04%; N, 14.04%; Actual value: C, 41.84%, H, 3.94%, N, 13.80%.
Embodiment 2
[0030] Embodiment 2: the preparation method of a kind of pyridine imidoxime iron catalyst of the present embodiment is carried out according to the following steps:
[0031] Under an argon atmosphere, first add anhydrous FeCl to a 25 mL Schlenk tube 2 (1.27mg, 10μmol, 1equiv.), then pyridineimine ligand L2 (3.0mg, 20μmol, 2equiv.) was added to the system, then 20mL of dichloromethane solvent was added, and the reaction was stirred at 25 ° C for 24h, the reaction was completed Afterwards, the filtrate was collected by filtration, concentrated to solid, washed with anhydrous n-hexane for 3 times, and dried in vacuum for 12 h to obtain iron pyridine imidoxime catalyst B (referred to as main catalyst B).
[0032] Mass spectrometry: C 14 H 20 Cl 2 FeN 4 O 2 : [M-Cl - ] + : theoretical value 391.0624, actual value: 391.0121.
[0033] Elemental Analysis: Theoretical: C, 44.99%; H, 4.72%; N, 13.12%; Actual: C, 45.34%; H, 4.51%; N, 13.40%.
Embodiment 3
[0034] Embodiment 3: the preparation method of a kind of pyridine imidoxime iron catalyst of the present embodiment is carried out according to the following steps:
[0035] Under an argon atmosphere, first add anhydrous FeCl to a 25 mL Schlenk tube 2 (1.27 mg, 10 μmol, 1 equiv.), then the pyridine imine ligand L3 (3.96 mg, 20 μmol, 2 equiv.) was added to the system, and then 20 mL of dichloromethane solvent was added, and the reaction was stirred at 25 ° C for 24 h, the reaction was completed After that, the filtrate was collected by filtration, concentrated to solid, washed with anhydrous n-hexane for 3 times, and dried in vacuum for 12 h to obtain iron pyridine imidoxime catalyst C (referred to as main catalyst C).
[0036] Mass spectrometry: C24 H 20 Cl 2 FeN 4 O 2 : [M-Cl - ] + : theoretical value 487.0624, actual value: 487.0421.
[0037] Elemental analysis: Theoretical value: C, 55.10%; H, 3.85%; N, 10.71%; Actual value: C, 54.91%; H, 3.56%; N, 11.13%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com