Alanine class compound, its preparing method and use
The technology of a compound, alanine, is applied in the field of medicinal chemistry and endocrine therapy, which can solve the problems of high liver toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
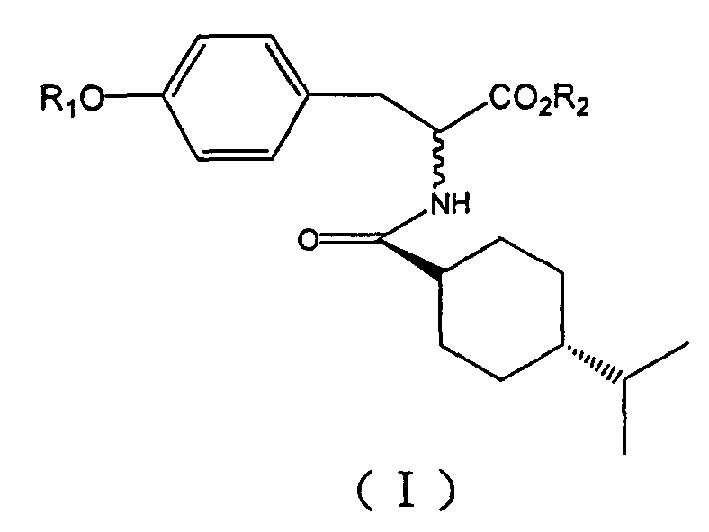
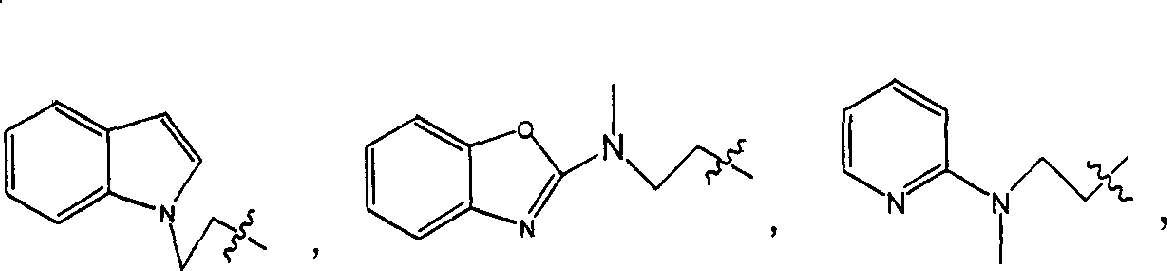
[0118] Example 1: (2S)-2-[N-(trans-4-isopropylcyclohexanecarbonyl)amino]-3-[4-[2-(5-methyl-2-phenyl- 4-oxazole)ethoxy]phenyl]propanoic acid (1)
[0119] (1) Condensation: 2-(5-methyl-2-phenyl-4-oxazole) ethanol 0.313g (1.54mmol) and (S)-B(2S)-2-[N-(trans-4 -Isopropylcyclohexanecarbonyl)amino]-3-(4-hydroxyphenyl)propionic acid methyl ester 0.535g (1.54mmol) in 30mL anhydrous tetrahydrofuran, add triphenylphosphine 0.605g (2.31mmol) , 370 μL (2.31 mmol) of diethyl azodicarboxylate was slowly added dropwise at 0°C. Stir at room temperature for 24 hours. The solvent was distilled off under reduced pressure, the residue was precipitated into a solid in ether, and a white solid was obtained by suction filtration. The solid was recrystallized from methanol to give (2S)-2-[N-(trans-4-isopropylcyclohexanecarbonyl)amino]-3-[4-[2-(5-methyl- 0.44 g of methyl 2-phenyl-4-oxazole)ethoxy]phenyl]propionate, yield 53.8%.
[0120] (2) Hydrolysis: Dissolve 0.26 g (0.5 mmol) of the product of...
Embodiment 2
[0126] Example 2: (2R)-2-[N-(trans-4-isopropylcyclohexanecarbonyl)amino]-3-[4-[2-(5-methyl-2-phenyl- 4-oxazole)ethoxy]phenyl]propanoic acid (2)
[0127] Take (2R)-2-[N-(trans-4-isopropylcyclohexanecarbonyl)amino]-3-(4-hydroxyphenyl)propionic acid methyl ester) ((R)-B) as Starting materials, the title compound was obtained by the same preparation method as in Example 1. m.p.151-153°C (decomposition). [α] D 25 -82.5(c, 0.217, CHCl 3 ); 1 H NMR is the same as the title compound of Example 1.
[0128] Elemental Analysis, C 31 h 38 N 2 o 5 (518):
[0129] Calculated C, 71.81; H, 7.34; N, 5.41;
[0130] Found C, 71.40; H, 8.16; N, 5.33.
[0131] IR(KBr): 3282.3, 2933.2, 2854.2, 1712.5, 1633.4, 1554.4, 1513.9, 1249.7, 1176.4, 715.5, 686.5cm -1 .
Embodiment 3
[0132] Example 3: (2S)-2-[N-(trans-4-isopropylcyclohexanecarbonyl)amino]-3-[4-[2-[N-methyl-N-(2- Benzoxazole) amino] ethoxy] phenyl] propanoic acid (3)
[0133]0.11 g (0.21 mmol) of compound D was dissolved in 2 ml of tetrahydrofuran, 240 μl (0.51 mmol) of triethylamine and 40 mg (0.26 mmol) of 2-chlorobenzoxazole were added. Stir at room temperature for 24 hours. Tetrahydrofuran was distilled off under reduced pressure, the residue was mixed with 4 ml of ethyl acetate, and the suspension was stirred evenly with 4 ml of saturated aqueous sodium bicarbonate solution. Stand to separate the ethyl acetate layer, and dry over anhydrous sodium sulfate. The residue was dissolved in a mixed solvent of petroleum ether: ethyl acetate = 1:1 to precipitate a white solid. The solid was hydrolyzed with lithium hydroxide to obtain 0.042 g of the target product, with a yield of 39.6%. m.p.179-180°C (decomposition). [α] D 25 81.1(c, 0.535, CHCl 3 );
[0134] 1 H NMR (DMSO): δ=0.81(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com