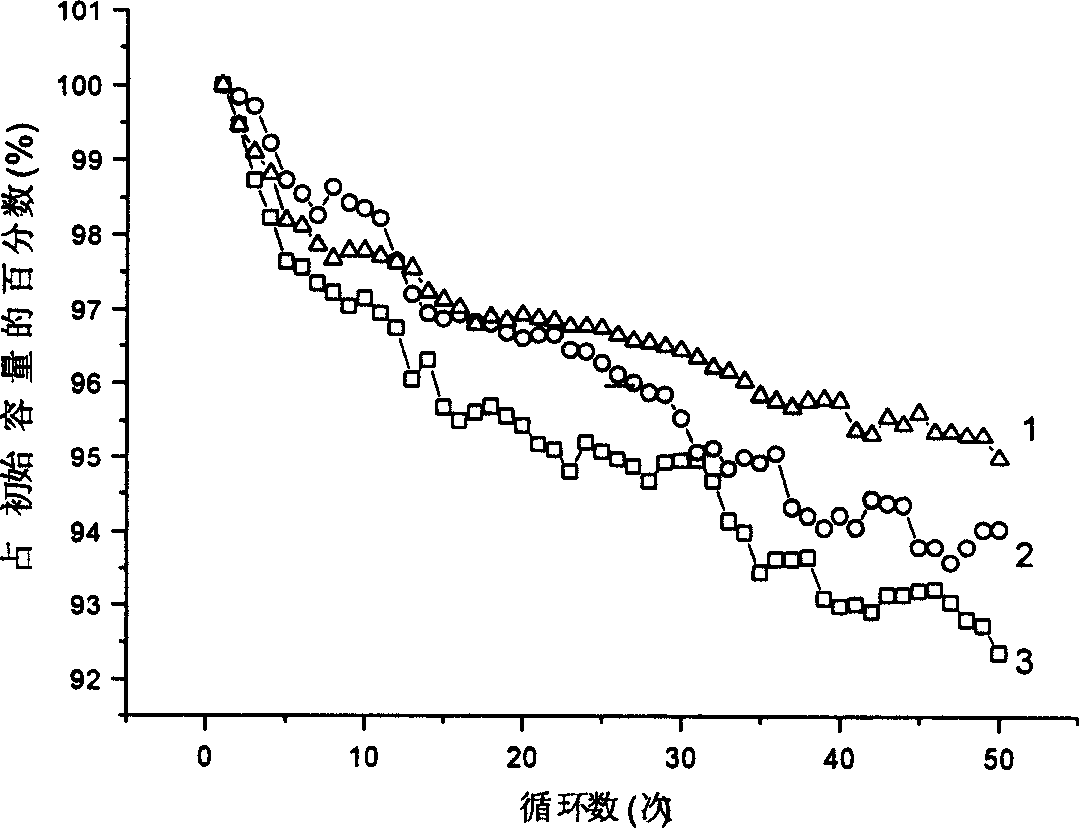
Method for preparing positive active material lithium cobaltate of lithium ion cell
A cathode active material, lithium-ion battery technology, applied in electrode manufacturing, battery electrodes, chemical instruments and methods, etc. Effects of chemical cycle performance, increased volumetric capacity, and reduced processing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Lithium carbonate and tricobalt tetroxide were mixed and ground in a ball mill for 3 hours according to the stoichiometric ratio Li: Co=1.02:1 (sample A) and Li: Co=1.08:1 (sample B), and then the homogeneously mixed materials were put into heating In the furnace, the heat treatment atmosphere is air, the temperature rise rate is 5°C / min to 700°C, after 2 hours of heat preservation, the temperature is raised to 800°C at 5°C / min, the heat treatment time is 5 hours, and then lowered to room temperature, and the synthetic material is crushed Sieve through a 300 mesh sieve.
[0024] Add sample A and sample B to the solution containing 5mmol / LMg at a solid-to-liquid ratio of 1:8, respectively. 2+ In the water of the precipitating agent (magnesium carbonate solution containing carbon dioxide), stir for 5 minutes, separate the solid and liquid after clarification, repeat the above washing operation until the pH of the supernatant is less than 8 (10% (W / V) LiCoO 2 pH value mea...
Embodiment 2
[0029] Lithium carbonate and cobalt carbonate were mixed in a ball mill for 3 hours according to the stoichiometric ratio Li:Co=1.02:1 (sample C) and Li:Co=1.08:1 (sample D), and then the uniformly mixed materials were put into In the heating furnace, the heat treatment atmosphere is oxygen, and the temperature rise rate is 5°C / min to 600°C. After 4 hours of heat preservation, the temperature is raised to 800°C at 5°C / min. The heat treatment time is 8 hours, and then lowered to room temperature, and the synthetic material After crushing, sieve through a 300-mesh sieve. Subsequent processing is the same as in Embodiment 1.
[0030] sample
[0031] Table 2. Comparison of several samples
Embodiment 3
[0033] According to the sample B obtained in Example 1, use Ca 2+ , Mg 2+ The concentration is 3mmol / L solution (calcium carbonate and magnesium carbonate solution containing carbon dioxide) treatment, solid-liquid ratio is 1:8. After washing twice, the pH of the upper layer solution is 7.8, and the pH value of lithium cobalt oxide after drying is 9.3; the discharge capacity of lithium cobalt oxide is 149mAh / g, and the tap density is 2.82g / cm 3 , specific surface area 0.37m 2 / g,D 50 is 11.5 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com