Process for preparing recombinant natookinase
A nattokinase and coding technology, which is applied to the coding nucleotide sequence of recombinant nattokinase and its preparation field, can solve the problems of low yield, high cost, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
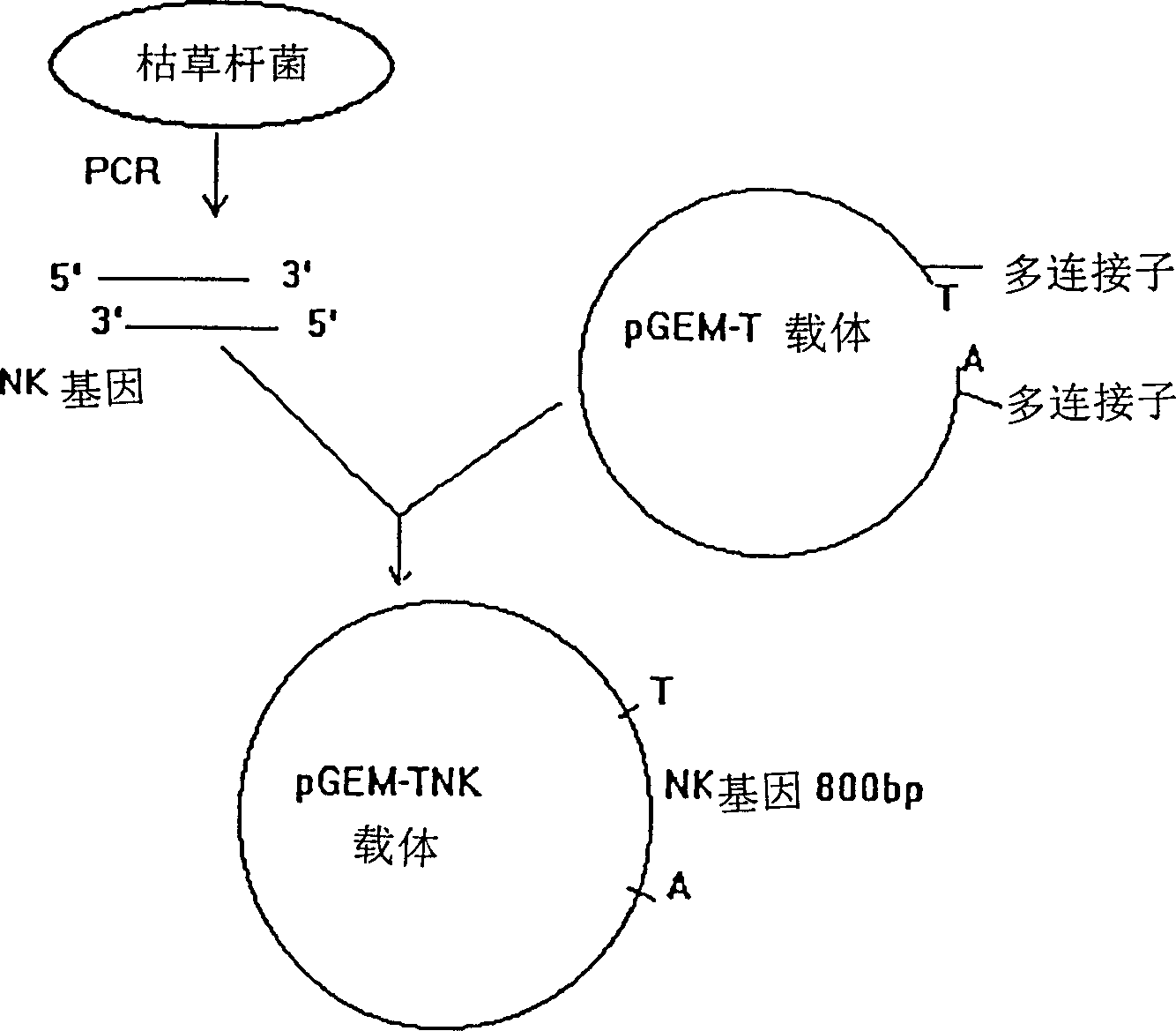
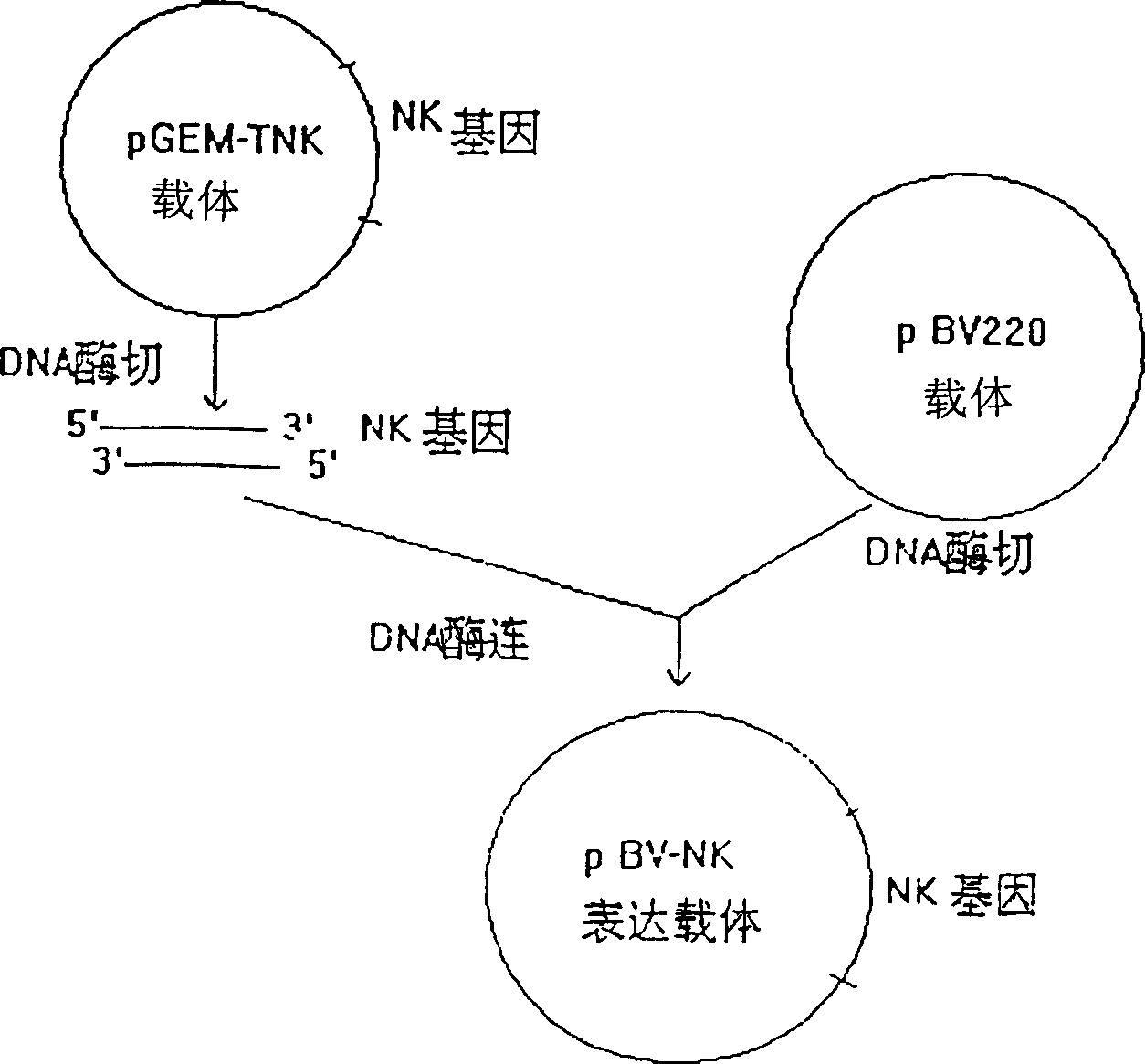
[0066] The construction and expression of embodiment one nattokinase gene expression vector:
[0067] 1. Extract total bacterial DNA from Bacillus subtilis notto: Inoculate Bacillus subtilis notto in LB medium, culture at 37°C and shake at 250rpm / min for 16 hours. Centrifuge at 500rpm / min for 10 minutes, collect the bacterial mass and resuspend in TE buffer, add SDS and proteinase K, and incubate at 37°C for 1 hour. Add NaCl, mix with CTAB, and incubate at 65°C for 10 minutes. Add an equal volume of chloroform / isoamyl alcohol for extraction, centrifuge and separate, and then extract the upper layer with an equal volume of phenol / chloroform / isoamyl alcohol. After centrifugation, add 0.6 times the volume of isoamyl alcohol to the upper layer, and centrifuge to precipitate. % ethanol was washed twice, drained and dissolved in TE buffer solution. Ultraviolet spectroscopic detection proved that A260 / A280>2.0.
[0068] 2. Amplify the nattokinase gene with the PCR method:
[0069]...
Embodiment 2
[0108] Embodiment two: large-scale preparation of genetically engineered nattokinase:
[0109] 1. Fermentation of engineering bacteria BVNK:
[0110] (1) Shake flask fermentation:
[0111] The monoclonal engineering bacteria BVNK was inoculated in LBG-ampicillin (100ug / ml) culture solution and cultured overnight at 30°C. The next day, it was diluted 1:50, shaken fully to OD600 0.4-0.6, and incubated at 42°C for 6 hours. The bacterial solution was centrifuged at 5000 rpm / min for 10 minutes at 4°C in a centrifuge tube used for weighing. The supernatant was discarded, and the pellet was washed once with PBS (pH 7.4), centrifuged and weighed.
[0112] (2) Fermentation tank (5L) fermentation:
[0113] BVNK monoclonal inoculation in 100ml LBG-ampicillin (100ug / ml) culture medium, cultivated overnight at 30°C, transferred to a fermenter at a ratio of 1:50 the next day, set the temperature at 30°C, pH6-8, dissolved oxygen 50%- 80%, the stirring speed is 600 r / min (stirring speed ...
Embodiment 3
[0130] Embodiment three uses the present invention to prepare recombinant nattokinase and carries out animal experiments:
[0131] 1. Acute toxicity test of recombinant nattokinase on mice.
[0132] 40 healthy mice, half male and half male, were randomly divided into 4 groups according to body weight. There were 10 mice in each group, dose interval K=0.48, different doses of drugs were injected into the tail vein of the mice respectively, the volume of administration was 0.5ml / 20kg body weight, and the reaction and death of the mice were observed after administration. According to BLISS statistical method to calculate LD50=8.54mg / kg
[0133] 2. Pharmacodynamic study of recombinant nattokinase
[0134] (1) The effect of intravenous injection of recombinant nattokinase on carotid thrombus formation in rats. 50 male rats were randomly divided into 5 groups, 10 rats in each group, respectively: Natrate recombinant soybean kinase large dosage group 6.0mg / kg, medium dosage group ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com