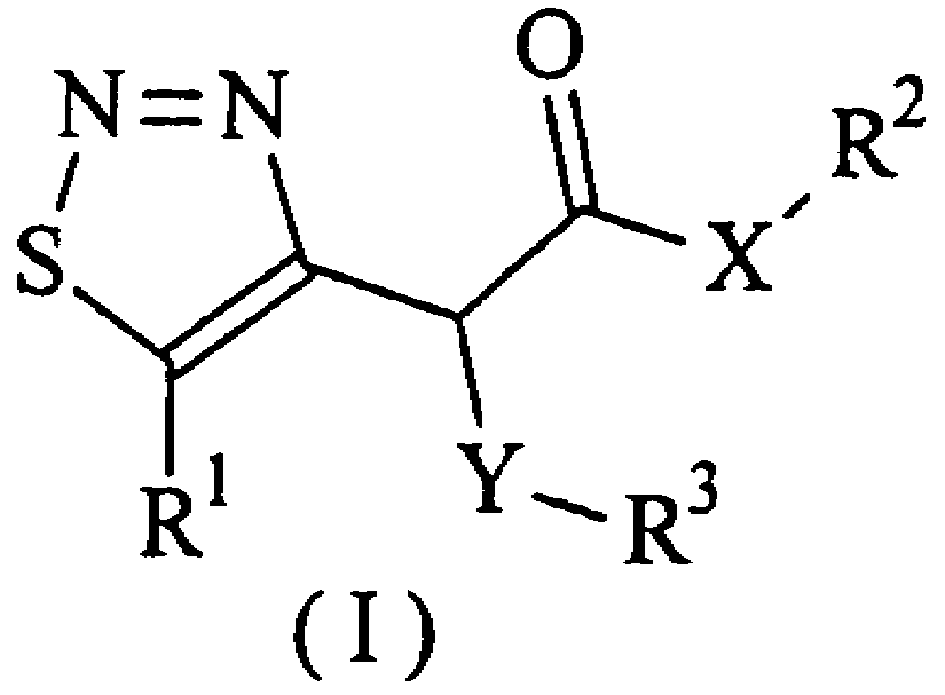
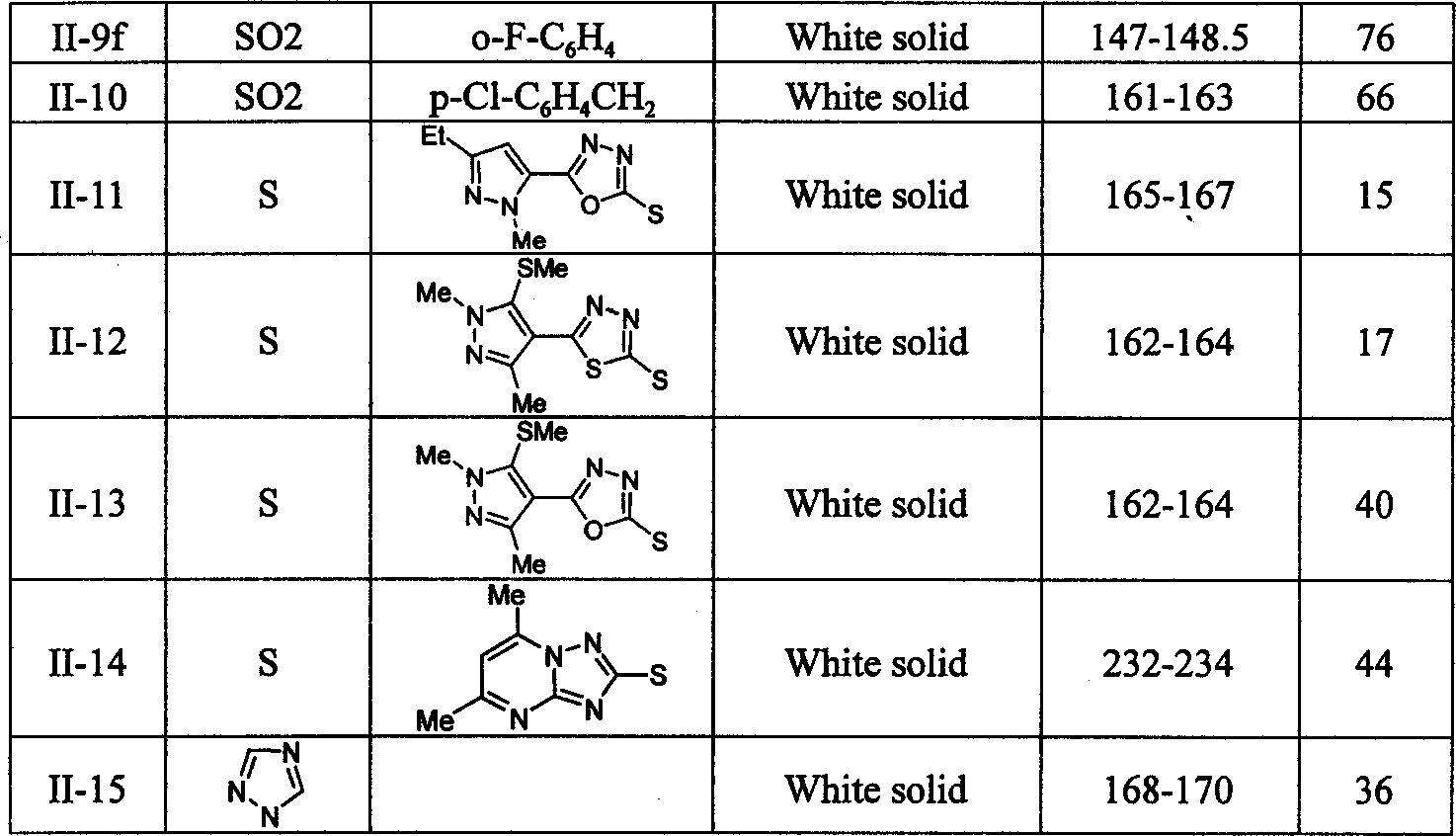
1,2,3-thiadiazole compounds, and their preparing process and bioactivity
A technology of thiadiazoles and compounds, which is applied in the field of preparation of thiadiazoles, can solve the problems of limited application, high cost, serious drug damage, etc., and achieve good inhibitory effect, novel structure, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of α-Chloroacetoacetylmethylamide-N-Ethoxycarbonylhydrazone
[0035] Dissolve 21 g (90%) (0.125 mol) of α-chloroacetoacetamide in 60 mL of absolute ethanol, slowly add 12.9 g (0.125 mol) of ethyl carbazate dropwise at room temperature, react at room temperature for 18 h, and remove in vacuo Part of the solvent was removed and recrystallized to obtain 21 g of a white solid with a yield of 71% and a melting point of 120-121°C. Elemental analysis: found, C: 40.78, H: 5.51, N: 17.64; calculated: C: 40.77, H: 5.99, N: 17.83. 1 H NMR (CDCl 3 ): 1.30 (t, J=7.2, 3H, CH 3 ), 1.90 (s, 3H, CH 3 ), 2.87 (d, J=4.6, 3H, NCH 3 ), 4.25 (q, J=7.0, 2H, OCH 2 ), 5.11 (s, 1H, CH), 6.76 (br, 1H, NH), 7.90 (br, 1H, NH).
Embodiment 2
[0037] Synthesis of α-chloro-1,2,3-thiadiazole acetamide
[0038] Add 25mL of thionyl chloride to the reaction flask, cool to 5°C, add 21g of the product from the previous step into the reaction flask in batches under stirring, and control the temperature not to exceed 10°C. In the sodium saturated aqueous solution, a large amount of solids were precipitated, filtered by suction, and recrystallized with ethanol to obtain 14.5 g of the product, the yield was 85%, and the melting point was 144-145°C. Elemental analysis: found, C: 31.45, H: 2.89, N: 21.77; calculated: C: 31.34, H: 3.16, N: 21.93. 1 HNMR (CDCl 3 ): 2.93 (d, J=4.8, 3H, NCH 3 ), 5.91 (s, 1H, CH), 6.96 (br, 1H, NH), 8.67 (s, 1H, thiadiazole-H).
Embodiment 3
[0040] Synthesis of α-substituted phenoxy-1,2,3-thiadiazole acetamide (II-3a)
[0041] Dissolve 0.87 g (4 mmol) of p-bromophenol in 10 mL of methanol, add 0.24 g (5 mmol) of sodium hydroxide, stir at room temperature for 10 minutes, then add 0.77 g of α-chloro-1,2,3-thiadiazole acetamide g (4 mmol), stirred at room temperature for 48 h, evaporated the solvent in vacuo, washed the solid with 10 mL of water, filtered with suction, and decompressed the residue on a silica gel column with eluent ethyl acetate / petroleum ether (v:v=1:5) Rinse and wash to obtain 0.6g of pure product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com