Hydrogen preparation method and apparatus
A hydrogen production device and hydrogen technology, applied in the field of electrochemistry, to achieve the effect of wide range of raw materials, small volume, and good catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
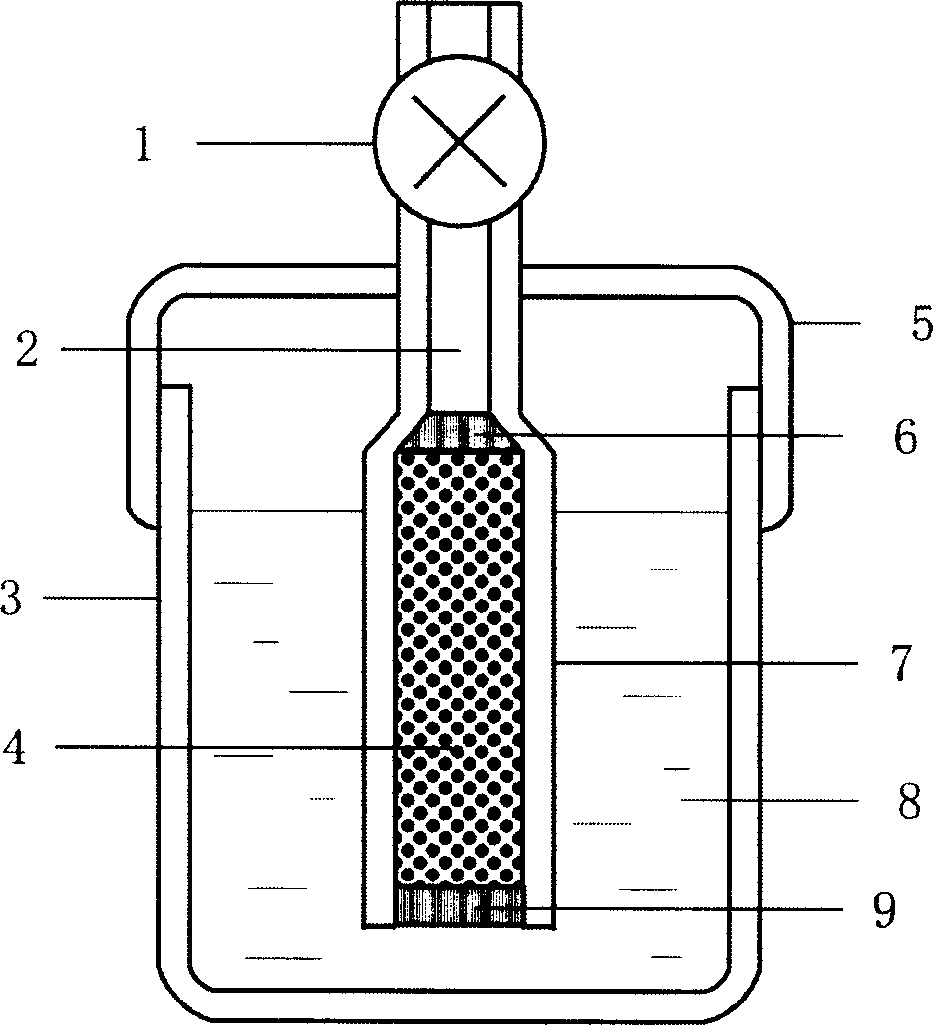
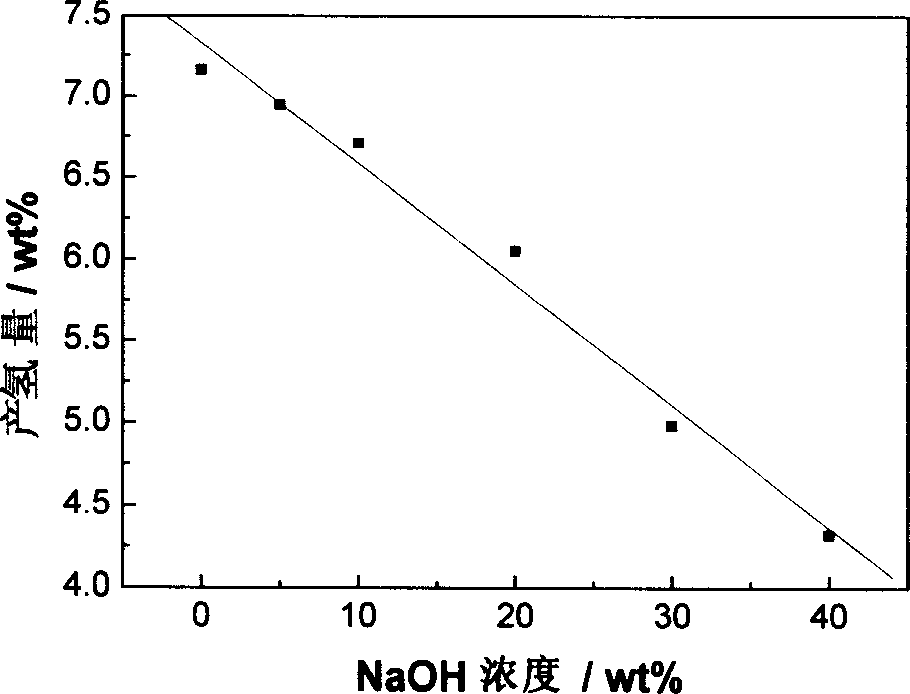
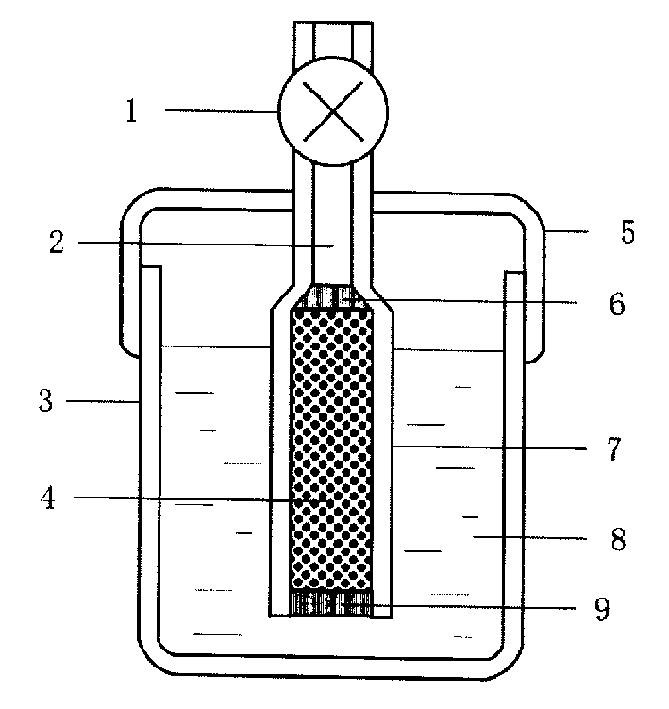
[0021] Embodiment 1: with 11.9g NiCl 2 ·6H 2 O is dissolved in twice distilled water and is made into 6wt% aqueous solution, adds 16g high specific surface activated carbon under constant stirring, is cooled to below 10 ℃ with mixture being placed in ice-water bath, then slowly drips the sodium borohydride aqueous solution of 5wt%, keeps The reaction temperature is still below 10°C. Nickel boride precipitates are formed through chemical redox reaction, which are evenly dispersed on the surface of the porous carrier. Drying for 24 hours under an inert atmosphere yields the desired catalyst of the present invention. The loading of the resulting highly dispersed catalyst was about 20 wt%.
Embodiment 2
[0022] Example 2: Dissolve 3.04g of ferric nitrate in twice-distilled water to form a 10wt% aqueous solution, add 0.3g of high specific surface acetylene black under constant stirring, place the mixture in an ice-water bath and cool it to 2°C, then slowly drop Add 5wt% potassium borohydride aqueous solution or alkaline aqueous solution, produce iron boride precipitation through chemical oxidation-reduction reaction, evenly disperse on the surface of porous carrier, after the reaction is complete, centrifugally filter and wash the loaded catalyst powder, and Dry in air to obtain the desired catalyst of the present invention. The loading of the resulting highly dispersed catalyst was about 80 wt%.
Embodiment 3
[0023] Example 3: Dissolve 6.0 g of cobalt sulfate in twice-distilled water to form a 30% solution, add about 25 g of β-alumina with a high specific surface area, stir for 0.5 h to make it evenly mixed, and place the mixture in an ice-water bath Cool to about 0°C, slowly add 11.4g of 20% (weight percent) sodium borohydride alkaline aqueous solution, and form a cobalt boride precipitate through a chemical oxidation-reduction reaction, which is evenly dispersed on the surface of β-alumina. After the reaction is complete, The loaded catalyst powder was filtered and dried at 250° C. for 12 hours under an argon atmosphere to obtain the desired catalyst of the present invention. The loading of the resulting highly dispersed catalyst was about 5 wt%.
[0024] Similar catalysts can be obtained by replacing the transition metal salts, metal borohydrides, and catalyst supports in the above examples with other transition metal salts, metal borohydrides, and catalyst supports described in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com