Method for preparing intermediate-tmeperature solid oxide electrolyte superfine powder for fuel cell
A solid oxide and fuel cell technology, applied in solid electrolyte fuel cells, fuel cells, oxide conductors, etc., can solve problems such as agglomeration of finished powder, difficult control of preparation conditions, and segregation of metal ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Example 1, La 0.9 Sr 0.1 Ga 0.8 Mg 0.2 o 3-δ (LSGM 9182) Synthesis of Ultrafine Powder
[0022] According to the stoichiometric ratio, La(NO 3 ) 3 ·6H 2 O, Sr(NO 3 ) 2 , Mg(NO 3 ) 2 ·6H 2 O and Ga 2 o 3 The amount of reagents, the above reagents are selected analytical grade reagents. The excess La(NO 3 ) 3 ·6H 2 O, Sr(NO 3 ) 2 , Mg(NO 3 ) 2 ·6H 2 O is dissolved in deionized water, and the molar concentration of the metal ions of each nitrate solution is accurately calibrated by chemical analysis (such as EDTA complexometric titration), and the volume of each nitrate solution required is calculated accordingly. Press the Calculate the amount to accurately weigh Ga 2 o 3 , and then dissolved in concentrated nitric acid, because Ga 2 o 3 It is relatively insoluble, so it must be heated with stirring long enough to ensure complete dissolution. Accurately measure La(NO 3 ) 3 ·6H 2 O, Sr(NO 3 ) 2 , Mg(NO 3 ) 2 ·6H 2 O solution, with Ga 2 o...
example 2
[0023] Example 2, La 0.9 Sr 0.1 Ga 0.8 Mg 0.2 o 3-δ (LSGM9182) Determination of roasting temperature of superfine powder, detection of particle size and electrical conductivity of synthetic powder
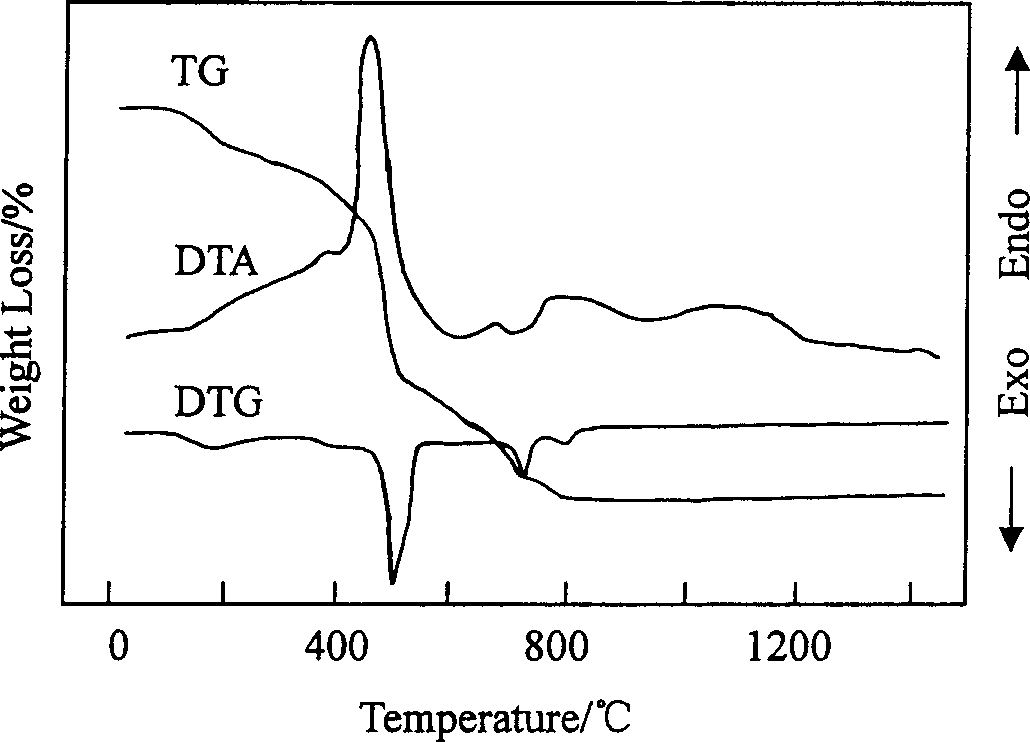
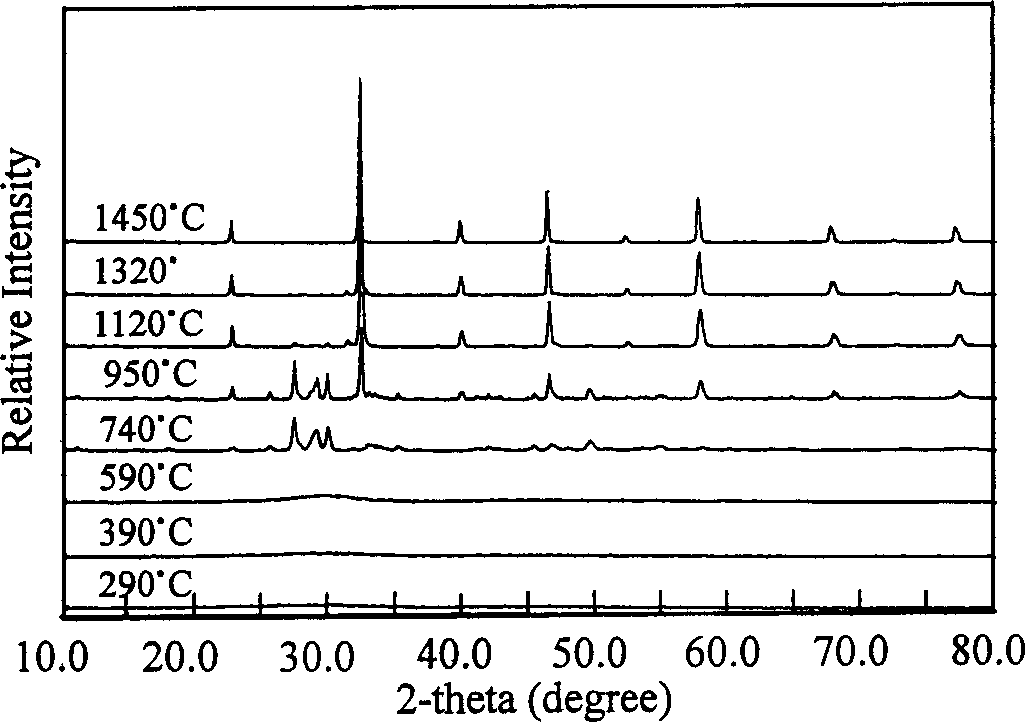
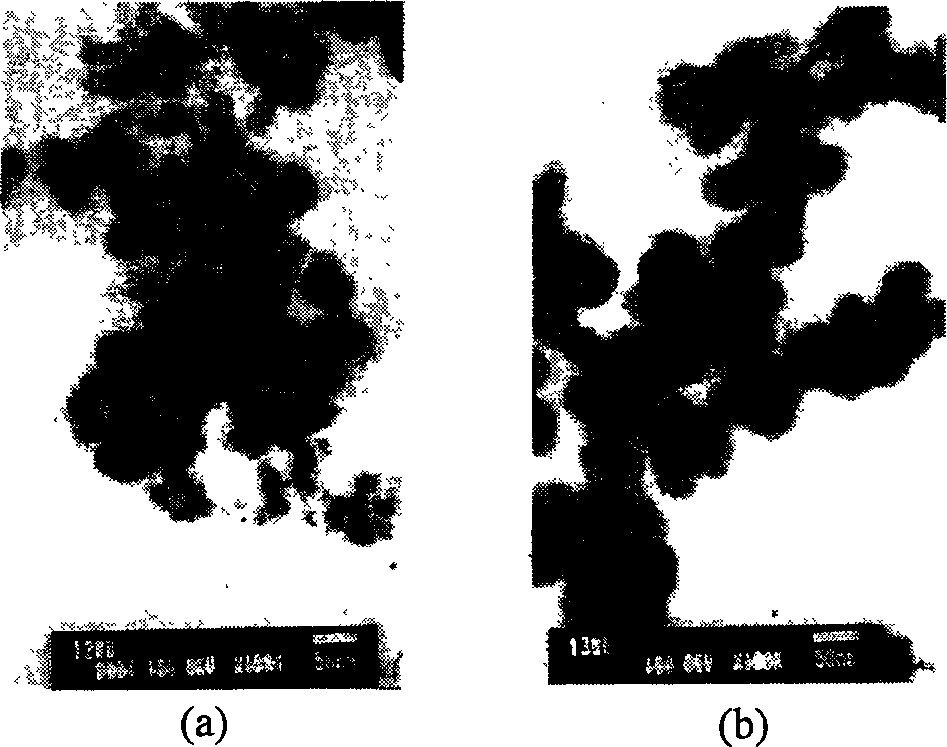
[0024] Use the differential thermal-thermogravimetric (TG-DTA) method to measure the mass change (weight loss) and heat absorption and heat release during the roasting process of the precursor powder, and preliminarily determine the changes in the precursor during the heating process, such as figure 1 shown. Then the precursor powder was calcined at different temperatures (290°C, 390°C, 590°C, 740°C, 950°C, 1120°C, 1320°C, 1450°C) for 180min, and the phase structure of the product was determined by X-ray diffraction method. The results are as follows: figure 2 shown. Finally, the precursor powder was roasted at 1200°C and 1300°C for 180min, and the morphology and particle size of the finished LSGM powder were observed with a transmission electron microscope (TEM), as shown in ...
example 3
[0027] Example 3, Sm 0.5 Sr 0.5 CoO 3-δ Synthesis of (SSC) Superfine Powder
[0028] According to the stoichiometric ratio, the Sr(NO 3 ) 2 , Co(NO 3 ) 3 ·6H 2 O and Sm 2 o 3 The amount of reagents, the above reagents are selected analytical grade reagents. Will Co(NO 3 ) 3 ·6H 2 O, Sr(NO 3 ) 2 , Dissolved in deionized water, using chemical analysis methods, such as EDTA complexometric titration, to accurately calibrate the molar concentration of metal ions in each nitrate solution, and calculate the required volume of each nitrate solution accordingly. Accurately weigh Sm according to the calculated amount with an analytical balance 2 o 3 , and then dissolved in concentrated nitric acid. Accurately measure Co(NO 3 ) 3 ·6H 2 O, Sr(NO 3 ) 2 solution, with Sm 2 o 3 The solution obtained by dissolving in nitric acid was mixed well. Weigh EDTA acid at a ratio of 1.2:1, add ammonia water until completely dissolved. Add this solution into the mixed solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com